|
|
| Line 1: |
Line 1: |
| − | ==Introduction== | + | == '''Objective''' == |
| | + | A global understanding of markets, products, and distribution channels related to the acne product market. Our overall focus is on the '''OTC market''', not prescription. |
| | | | |
| − | Over-the-counter (OTC) drugs are medicines that may be sold directly to a consumer without a prescription from a healthcare professional, and are commonly used to treat symptoms of common illnesses that may not require the direct supervision of a physician. <br>
| + | == '''Introduction''' == |
| − | *For a medicine to be granted OTC status, it must have a wide safety margin and be effective, and must bear understandable labeling to ensure proper use
| + | |
| − | *More than 700 OTC products on the market today use ingredients or dosages, that were available only by prescription, less than 30 years ago
| + | |
| − | *Rx to OTC switch refers to the transfer of proven prescription drugs (Rx) to non-prescription, over-the-counter (OTC) status. Rx to OTC switch is a data-driven, scientifically rigorous, and highly regulated process that allows consumers to have OTC access to a growing range of medicines
| + | |
| − | <br>
| + | |
| − | There are two ways in which drugs are commonly switched as approved by FDA in US :
| + | |
| | | | |
| − | #The OTC Drug Review
| + | '''Definition''' |
| − | #*Began in 1972
| + | |
| − | #*Ongoing assessment of the safety and effectiveness of all nonprescription drugs
| + | |
| − | #*Panels of non-government experts review active ingredients in marketed OTC drug products to determine whether they can be classified as safe and effective
| + | |
| − | #*About 40 former prescription-only drug ingredients have been switched by this process
| + | |
| − | #New drug application (NDA) process
| + | |
| − | #*Manufacturers submit data to the FDA showing the drug is appropriate for self-administration.
| + | |
| − | #*The submission includes studies showing that the product's labeling can be read, understood, and followed by the consumer without the guidance of a health care provider
| + | |
| − | #*Some drugs are approved initially as OTC drugs, but most are first approved for prescription use and later switched to OTC
| + | |
| | | | |
| − | ==Rx and OTC Regulation==
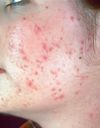
| + | Acne vulgaris (commonly called acne) is a common skin condition, affecting 85-100% of people at some time during their lives. It is caused by changes in the pilosebaceous units, skin structures consisting of a hair follicle and its associated sebaceous gland. It is characterized by noninflammatory follicular papules or comedones and by inflammatory papules, pustules, and nodules in its more severe forms. Acne vulgaris affects the areas of skin with the densest population of sebaceous follicles; these areas include the face, the upper part of the chest, and the back. Severe acne is inflammatory, but acne can also manifest in noninflammatory forms. Acne lesions are commonly referred to as pimples, blemishes, spots, zits, or acne. [http://en.wikipedia.org/wiki/Acne_vulgaris Source] |
| | | | |
| − | The FDA process for drug approval is given below
| + | [[Image:acne.jpg|thumb|100px|right|[http://www.dermatology.svhm.org.au/Primary%20School/acne.htm Source]]] |
| | | | |
| − | [[Image:FDA Drug Regulation Process.jpg |center|thumb|1100px| [http://www.fda.gov/Drugs/default.htm FDA Drug Regulation Process]]]
| + | There's been a gap in products marketed to adult women--little or nothing was available to address their acne needs, and as a result, sales have lagged. "No one was looking to target the 30- to 40-year-olds," said Lee Feldman, vice president of sales for Advanced Research Labs. [http://findarticles.com/p/articles/mi_m3374/is_2_21/ai_53748892 Source] |
| | | | |
| − | * NDA - New Drug Application | + | * In this report we have presented Acne Market details for following countries. |
| − | * ANDA - Abbreviated New Drug Application
| + | |
| − | * Full NDA- For a new dosage or formulation such as lower strength than Rx version (for a medication currently available by prescription)
| + | |
| − | * Supplemental NDA – For a product for which the manufacturer already holds an approved NDA or holds an abbreviated NDA for a closely related product
| + | |
| − | * Abbreviated NDA – For products that are identical to an existing prescription product
| + | |
| − | * The switch to OTC is done in a partial or total way. Most switches are partial, a version of the active ingredient remains available on prescription while a specific indication, strength, or dosage form becomes available through the(new NDA) switch application. On the other hand some switches are full, no prescription version of the active ingredient remains which becomes available through (NDA supplement) switch application.
| + | |
| | | | |
| − | Source:[http://www.fda.gov FDA] , [http://books.google.co.in/books?id=XU1sMK1djVAC&pg=PA22&lpg=PA22&dq=rx-to-otc+switch+full+partial&source=bl&ots=o32Y2RGGOL&sig=6bYHqWf8Qi79E1Tq7-bm340hJ7A&hl=en&sa=X&ei=e3LCUvCiC8WwiQeeg4HgDA&redir_esc=y#v=onepage&q=rx-to-otc%20switch%20full%20partial&f=false Nonprescription Product Therapeutics, Section 1, Chapter 2]
| + | === Causes of Acne === |
| − | ===Prescription Drugs - Branded===
| + | |
| − | Once a drug has been discovered, the drug manufacturer must test the drug in laboratory and on animals. If it proves effective, the drug is tested in humans to see if it safe and effective.
| + | |
| − | Once it is demonstrated to be safe, the manufacturer sends a '''New Drug Application (NDA)''' to the FDA to apply for approval. Next come tests in humans to see if the drug is safe and effective when used to treat or diagnose a disease.
| + | |
| | | | |
| − | '''New Drug Application (NDA)'''
| + | Acne is a common occurrence at puberty. With the increased production of sex hormones, the sebaceous glands become hyperactive. |
| − | <br>
| + | Androgen and progesterone are responsible for the hyperplasia of the oil glands. |
| − | The NDA application is the vehicle through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale and marketing in the U.S. The data gathered during the animal studies and human clinical trials of an Investigational New Drug (IND) become part of the NDA.
| + | The usual premenstrual flare-up is explained by some observers as occurring during the period when the normal androgen-estrogen balance in the blood is altered in favor of androgen. Hence hormonal imbalance is held responsible for causing acne. |
| − | The goals of the NDA are to provide enough information to permit FDA reviewer to reach the following key decisions:
| + | Psychogenic stresses particularly the habit of picking pimples, makes them worse. |
| − | #Whether the drug is safe and effective in its proposed use(s), and whether the benefits of the drug outweigh the risks.
| + | |
| − | #Whether the drug's proposed labeling (package insert) is appropriate, and what it should contain.
| + | |
| − | #Whether the methods used in manufacturing the drug and the controls used to maintain the drug's quality are adequate to preserve the drug's identity, strength, quality, and purity.
| + | |
| − | The documentation required in an NDA is supposed to tell the drug's whole story, including what happened during the clinical tests, what the ingredients of the drug are, the results of the animal studies, how the drug behaves in the body, and how it is manufactured, processed and packaged. | + | |
| − | <br>
| + | |
| − | NDAs include:
| + | |
| − | #The drug's test results
| + | |
| − | #Manufacturing information to demonstrate the company can properly manufacture the drug.
| + | |
| − | #The company's proposed label for the drug, which should include information about the drug, its uses, and possible risks.
| + | |
| − | The FDA approves drugs if their benefits outweigh any risks in taking them. Once a drug is approved, its manufacturer can market and then sell it in the U.S.
| + | |
| − | In approving the drug, the FDA also takes into account the proposed name for it. To protect the safety of the public, it is important that the name not be similar to that of another drug product to prevent against mix-ups. FDA's Division of Medication Error Prevention and Analysis reviews the names.
| + | |
| | | | |
| − | Source:[http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/NewDrugApplicationNDA/default.htm FDA],[http://pharmacy.about.com/od/TheDrugIndustry/a/The-Fda-Drug-Approval-Process.htm Pharmacy.About]
| + | Besides seborrhoeic diathesis and hormonal imbalance, other factors which aggravate acne are: |
| − | <br>
| + | |
| | | | |
| − | ===Prescription Drugs – Generic===
| + | * a diet rich in fats and starches; |
| − | Generic drugs are also known as copycat drugs. They are exact replicas of the branded drugs and typically go by their chemical name-Lipitor's generic is called atorvastatin, for example. They do not have a patent.
| + | * intestinal stasis, especially constipation; |
| − | The manufacturers of generic drugs provide an '''abbreviated new drug application (ANDA)''' to the FDA to receive approval. Since there is already a drug on the market to help consumers, the approval time for generic drugs is typically longer.
| + | * a sedentary life; |
| − | The Office of Generic Drugs (OGD), which is a department within the FDA, approves generic drugs.
| + | * excessive use of greasy cosmetics, pomade, detergents and mechanical rubbing |
| | + | * Heavy or oily make up |
| | + | * Over abrasive cleansing |
| | + | * Diet high in nutritious food can also help aggravate acne. |
| | + | [http://www.acne-psoriasis-treatment.com/acne/acne-cause.htm Source] |
| | | | |
| − | An Abbreviated New Drug Application (ANDA) is submitted to the FDA when seeking review and approval for a generic drug product. The application is submitted to the FDA's Center for Drug Evaluation and Research, Office of Generic Drugs. If approved, the generic drug may be manufactured and sold in the U.S. market.
| + | === Acne Types === |
| − | Generic drug applications are not typically required to include preclinical (animal) and clinical (human) data to establish safety and effectiveness. An applicant submitting an ANDA must scientifically demonstrate that its product is bioequivalent to the previously approved innovator or brand name drug.
| + | There are various types of acne and they are:- |
| − | <br>
| + | |
| − | Source:[http://pharmacy.about.com/od/TheDrugIndustry/a/The-Fda-Drug-Approval-Process.htm Pharmacy.about1], [http://pharma.about.com/od/A/g/Abbreviated-New-Drug-Application.htm Pharmacy.about2]
| + | |
| | | | |
| − | FDA requires generic drugs to have the same quality and performance as brand name drugs.
| + | * Acne Rosacea |
| − | #When a generic drug product is approved, it has met rigorous standards established by the FDA with respect to identity, strength, quality, purity, and potency. However, some variability can and does occur during manufacturing, for both brand name and generic drugs. When a drug, generic or brand name, is mass-produced, very small variations in purity, size, strength, and other parameters are permitted. FDA limits how much variability is acceptable.
| + | * Acne Cosmetica |
| − | #Generic drugs are required to have the same active ingredient, strength, dosage form, and route of administration as the brand name product. Generic drugs do not need to contain the same inactive ingredients as the brand name product.
| + | * Acne Vulgaris |
| − | #The generic drug manufacturer must prove its drug is the same as (bioequivalent) the brand name drug. For example, after the patient takes the generic drug, the amount of drug in the bloodstream is measured. If the levels of the drug in the bloodstream are the same as the levels found when the brand name product is used, the generic drug will work the same.
| + | * Acne Fulminans |
| − | #Through review of bioequivalence data, FDA ensures that the generic product performs the same as its respective brand name product. This standard applies to all generic drugs, whether immediate or controlled release.
| + | * Acne Keloidalis Nuchae |
| − | #All generic manufacturing, packaging, and testing sites must pass the same quality standards as those of brand name drugs, and the generic products must meet the same exacting specifications as any brand name product. In fact, many generic drugs are made in the same manufacturing plants as brand name drug products.
| + | * Acne Chloracne |
| | + | * Acne Medicamentosa |
| | + | [http://www.acne-psoriasis-treatment.com/acne/acne-types.html Source] |
| | | | |
| − | Source: [http://www.fda.gov/drugs/resourcesforyou/consumers/buyingusingmedicinesafely/understandinggenericdrugs/ucm167991.htm FDA]
| + | === Acne Treatment === |
| − | <br>
| + | |
| | | | |
| − | ===OTC - Branded & Prescription Drugs===
| + | '''Medical Treatment''' |
| − | The FDA's '''Office of Drug Evaluation IV''', Over-the-Counter Drug Products reviews OTC drugs. A Nonprescription Drug Advisory Committee, comprised of up to 14 independent experts selected by the FDA commissioner, assists the FDA in reviewing issues surrounding OTC drugs, including switching drugs from prescription to non-prescription status.
| + | |
| | | | |
| − | Instead of reviewing the labels and ingredients of all 300,000 OTC drugs now being marketed, the FDA focuses on about 80 different therapeutic classes of drugs, such as analgesics, antacids, antimicrobial, antiperspirants, dental and cough/cold medicines.
| + | Medical treatment is suggested only in cases of chronic or severe acne and should be taken from the dermatologist. |
| | | | |
| − | The FDA publishes an '''OTC drug monograph''' for each category of drug in the Federal Register. Drug monographs serve as a cook book, or list of recipes, that include acceptable ingredients, formulations, dosages and labeling for each category of drug.The monograph explains the types of ingredients that may be used to treat certain diseases or conditions without a prescription, the appropriate dose and instructions for use, and labeling.
| + | To outline the drug treatment, it aims chiefly to: |
| | | | |
| − | Once a monograph is adopted, companies may develop and market an OTC product without pre-approval from the FDA. Products that do not adhere to the monograph must be reviewed through FDA’s '''New Drug Application''' process.A drug company can also petition the FDA to deviate from the ingredient or labeling requirements of an OTC monograph.
| + | * Cut down the bacteria in the hair follicles and on the skin surface. |
| | + | * Open the hair follicles by removal of the blackheads. |
| | + | * Cut down the acne inflammations. |
| | | | |
| − | #Over-the-counter (OTC) drugs are drugs that the FDA has decided are safe and appropriate for use without the supervision of a health care professional and can be purchased without a prescription.
| + | '''Local Drug Treatments''' |
| − | #Products conforming to a monograph may be marketed without further FDA clearance, while those that do not must undergo separate review and approval through the "New Drug Approval System".
| + | |
| − | #OTC products that meet a monograph's requirements may be marketed without FDA review. OTC products that do not fit under an existing monograph must be approved under an application like the '''applications for prescription products'''.
| + | |
| | | | |
| − | Source:[http://pharma.about.com/od/Over-the-Counter-Medicine/a/Over-The-Counter-Drugs-Theres-A-Recipe-For-That.htm Pharmacy.About1],[http://pharmacy.about.com/od/TheDrugIndustry/a/The-Fda-Drug-Approval-Process.htm Pharmacy.About2]
| + | Benzoyl Peroxide preparations available as gels and lotions have become very popular in the treatment of acne. Cream "Ultra Clearasil" is a very potent Benzoyl preparation. One of the main action points of Benzoyl Peroxide is to kill bacteria. It releases oxygen which helps in killing aerobic bacteria. |
| | | | |
| − | ===Rx to OTC Switch – Regulatory Process===
| + | The sulphur drug preparations, available as creams, gels and lotions, are extremely effective in treating acne. Like Benzoyl Peroxide, sulphur is committed to killing bacteria. Sulphur is a disinfectant and used in skin treatments for eczema, etc. |
| | | | |
| − | There are 2 regulatory pathways for getting approval for OTC drugs (Rx to OTC switch and Direct to OTC)
| + | Tetracycline usually has no side-effects and hence is the safest drug. However, in some uncommon cases, it causes bilousness, indigestion, vaginal thrush or rash. |
| − |
| + | |
| − | # OTC New Drug Application (NDA)
| + | |
| − | # OTC Drug Monograph
| + | |
| | | | |
| − | '''Rx-to-OTC switches'''
| + | [http://www.acne-psoriasis-treatment.com/acne/acne-treatment.html Source] |
| | | | |
| − | The applications differ based on whether the manufacturer wants to switch to OTC completely or partially.
| + | == Research Methodology == |
| − | #Full switch (NDA supplement)
| + | |
| − | #Partial switch (new NDA)
| + | |
| − | Source:[http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/UCM148055.pdf FDA]
| + | |
| | | | |
| − | '''Direct-to-OTC''' | + | '''Objective''' |
| − | #OTC Monographs
| + | A global understanding of markets, products, and distribution channels related to the acne product market. Our overall focus is on the OTC market(Excluding prescription drugs). |
| − | #OTC NDA
| + | |
| − | Source:[http://www.slideshare.net/nkp8490/an-overveiw-on-regulation-of-otc-drug-product-in-different-country OTC Regulation in Different Countries]
| + | |
| | | | |
| − | '''FDA Applications''' | + | '''Data Collection''' |
| | | | |
| − | #An efficacy supplement should be submitted to an approved NDA for a prescription product if the sponsor plans to switch the drug product covered under the NDA to OTC marketing status in its entirety without a change in the previously approved dosage form or route of administration.
| + | Secondary data sources are only used to conduct the research. |
| − | #An NDA 505(b)(1) should be submitted if the sponsor is proposing to convert some but not all of the approved prescription indications to OTC marketing status.
| + | |
| − | #An original NDA (505)(b)(1) or 505(b)(2) needs to be submitted if the sponsor plans to market either a new product OTC whose active substance, indication, or dosage form has never previously been marketed OTC.
| + | |
| | | | |
| − | '''OTC drug review'''
| + | The list of various sources used to conduct the research are as follows: |
| | | | |
| − | The OTC drug review was established to evaluate the safety and effectiveness of OTC drug products marketed in the United States before May 11, 1972. It is a three-phase public rulemaking process (each phase requiring a Federal Register publication) resulting in the establishment of standards (monographs or non-monographs) for an OTC therapeutic drug category.
| + | # [http://www.euromonitor.com Euromonmitor International] |
| | + | # [http://www.bccresearch.com BCC Research] |
| | + | # [http://www.ibisworld.com/ IBISWorld Industry Research] |
| | + | # [http://www.marketresearch.com MarketResearch.com] |
| | + | # [http://www.frost.com/prod/servlet/frost-home.pag Frost and Sullivan] |
| | + | # [http://www.marketlineinfo.com Marketline Business Information Center (Datamonitor)] |
| | + | # Online Pharmacy Store: [http://international.drugstore.com/default.asp drugstore.com] |
| | + | # Search Engine: Google |
| | | | |
| − | ::'''First phase'''
| + | '''Scope of the study''' |
| − | :::The first phase was accomplished by advisory review panels. The panels were charged with reviewing the ingredients in nonprescription drug products to determine whether these ingredients could be generally recognized as safe and effective for use in self-treatment. They were also charged with reviewing claims and recommending appropriate labeling, including therapeutic indications, dosage instructions, and warnings about side effects and preventing misuse.
| + | |
| − | :::According to the terms of the review, the panels classified ingredients in three categories as follows:
| + | |
| − | :::#Category I: generally recognized as safe and effective for the claimed therapeutic indication;
| + | |
| − | :::#Category II: not generally recognized as safe and effective or unacceptable indications;
| + | |
| − | :::#Category III: insufficient data available to permit final classification
| + | |
| | | | |
| − | ::'''Second phase'''
| + | # Included two major markets namely U S and Japan. |
| − | :::The second phase of the OTC drug review was the agency’s review of ingredients in each class of drugs, based on the panel’s findings, on public comment, and on new data that may have become available. The agency, in turn, publishes its conclusions in the Federal Register in the form of a tentative final monograph. After publication of the tentative final monograph, a period of time is allotted for objections to the agency’s proposal or for requests to be submitted for a hearing before the Commissioner of FDA.
| + | # Study covers leading brands for Acne treatment for OTC and not Prescription drugs for mass distribution channel that includes mainstream supermarkets, chain drugstores and mass merchandisers. |
| | | | |
| − | ::'''Third phase'''
| + | =Global Acne Medications Market= |
| − | :::The publication of final regulations in the form of drug monographs is the third and last phase of the review process. The monographs establish conditions under which certain OTC drug products are generally recognized as safe and effective.
| + | |
| | | | |
| − | ===Clinical Trials : Rx-to-OTC===
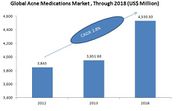
| + | As per BCC Research the value of the Global Acne Medications Market for year 2012 was estimated to be $3.5 billion and it is expected to reach $4.5 billion by year 2018 at a CAGR of 2.8%. |
| − |
| + | |
| − | Clinical trials are not a compulsory requirement for Rx-to-OTC switches, but most switch applications include new clinical trial data for the non-prescription indication and almost all the switch applications include label comprehension studies and/or actual use studies to demonstrate
| + | |
| − | that the medicine can be used safely and effectively in the consumer target population. Additional standard efficacy and safety clinical trials are to prove the drug can be used safely in an OTC setting.
| + | |
| − |
| + | |
| − | FDA’s Center for Drug Evaluation and Research (CDER) defines OTC drug actual use study as “a controlled experiment in which a prescription drug or unapproved new drug is used by subjects under OTC-like conditions.” OTC studies are intended to support a significant change in labelling for the drug. These studies are considered the most important in assessing a drug’s appropriateness for a switch. The main Objectives of an Rx-to OTC study fall into four main categories:
| + | |
| | | | |
| − | # Safety (prescription safety demonstrated by previous trials)
| |
| − | # Comprehension (demonstrated by label comprehension studies)
| |
| − | # Self-selection and de-selection
| |
| − | # Compliance as the core issue
| |
| | | | |
| − | If the drug has a good safety profile, shown by the studies done to support its marketing as a prescription drug, and if the drug meets the specific criteria for switching which includes ability of selection or de-selection by consumer, there is relatively good chance that FDA will approve it for OTC use.
| + | [[Image:BCC ACNE.jpg|center|thumb|center|632px * 341px|Source:BCC Research,[http://www.marketresearch.com/BCC-Research-v374/Skin-Disease-Treatment-Technologies-Global-7616658]]]] |
| − | | + | Source: [http://www.marketresearch.com/BCC-Research-v374/Skin-Disease-Treatment-Technologies-Global-7616658/ BCC Research] |
| − | Source:[http://www.fda.gov/Drugs/DevelopmentApprovalProcess/SmallBusinessAssistance/ucm069917.htm FDA], [http://www.complianceonline.com/articlefiles/Regulatory_Requirements_Prescription_to_OTC_Switches_USA_India.pdf Compliance Online] | + | {|border="0" cellspacing="0" cellpadding = "0" width="100%" |
| − | | + | |align = "center"|<font color="#282828"><font size = "4">'''The Acne Product Pipeline'''</font></font> |
| − | ==Market Overview==
| + | |
| − | ===OTC Market===
| + | |
| − | [[Image:OTC Market.png|center|thumb|900px|Source:Kalorama Information]]
| + | |
| − | {|border="2" align="center" cellspacing="0" cellpadding="4" width="28%" | + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Time Period''' | + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''CAGR'''
| + | |
| − | |-
| + | |
| − | |align = "center"|2005-2009
| + | |
| − | |align = "center"|3.3%
| + | |
| − | |-
| + | |
| − | |align = "center"|2009-2014
| + | |
| − | |align = "center"|2.7%
| + | |
| − | |-
| + | |
| − | |align = "center"|2005-2014
| + | |
| − | |align = "center"|3.0%
| + | |
| | |- | | |- |
| | |} | | |} |
| − | <br>
| |
| | | | |
| − | ===Rx vs. OTC Market===
| + | |
| − | '''Total Pharmaceutical Market by Country, 2009 ($B)'''
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" align="center" |
| − | {|border="2" cellspacing="0" cellpadding="4" width="100%" | + | |align = "center"|<br>''' ''' '''Product''' |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Country'''
| + | |align = "center"|''' '''<br>'''Company''' |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Rx Market'''
| + | |align = "center"|<br>''' Indication''' |
| − | |align = "center" bgcolor = "#8DB4E3"|'''OTC Market''' | + | |align = "center"|<br>'''Development Status''' |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Total Pharma Market''' | + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''OTC as % of Total Pharma Market''' | + | |
| | |- | | |- |
| − | |align = "center" bgcolor = "#DBE5F1"|'''United States''' | + | |ACAT Inhibitor |
| − | |align = "center" bgcolor = "#DBE5F1"|214 | + | |Graceway Pharmaceuticals |
| − | |align = "center" bgcolor = "#DBE5F1"|18 | + | |Acne |
| − | |align = "center" bgcolor = "#DBE5F1"|232 | + | |Phase II |
| − | |align = "center" bgcolor = "#DBE5F1"|'''7.8'''
| + | |
| | |- | | |- |
| − | |align = "center"|'''Japan''' | + | |ANT-1207 (botulinum toxin A) |
| − | |align = "center"|56 | + | |Anterios |
| − | |align = "center"|11 | + | |Acne vulgaris |
| − | |align = "center"|67 | + | |Phase II |
| − | |align = "center"|'''16.4'''
| + | |
| | |- | | |- |
| − | |align = "center"|'''Germany''' | + | |ASC-J9 |
| − | |align = "center"|35 | + | |AndroScience |
| − | |align = "center"|5 | + | |Acne vulgaris |
| − | |align = "center"|40 | + | |Phase II |
| − | |align = "center"|'''12.5'''
| + | |
| | |- | | |- |
| − | |align = "center"|'''France''' | + | |AUS-131/doxycycline |
| − | |align = "center"|32 | + | |Nexgen Dermatologics |
| − | |align = "center"|4 | + | |Acne vulgaris |
| − | |align = "center"|36 | + | |Phase I/II |
| − | |align = "center"|'''11.1'''
| + | |
| | |- | | |- |
| − | |align = "center"|'''China''' | + | |BLI-1100 |
| − | |align = "center"|16 | + | |Braintree Laboratories |
| − | |align = "center"|5 | + | |Acne vulgaris |
| − | |align = "center"|21 | + | |Phase II |
| − | |align = "center"|'''23.1'''
| + | |
| | |- | | |- |
| − | |align = "center"|'''United Kingdom''' | + | |CD-07223 |
| − | |align = "center"|17 | + | |Galderma R &D |
| − | |align = "center"|3 | + | |Acne |
| − | |align = "center"|20 | + | |Phase II |
| − | |align = "center"|'''15.0'''
| + | |
| | |- | | |- |
| − | |align = "center"|'''Russia''' | + | |CD-2475-101 |
| − | |align = "center"|13 | + | |Galderma Laboratories |
| − | |align = "center"|3 | + | |Acne vulgaris |
| − | |align = "center"|16 | + | |Phase I |
| − | |align = "center"|'''18.8'''
| + | |
| | |- | | |- |
| − | |align = "center"|'''Brazil''' | + | |Clindamycin/tretinoin topical combination |
| − | |align = "center"|10 | + | |Skinvisible Pharmaceuticals |
| − | |align = "center"|2 | + | |Acne |
| − | |align = "center"|12 | + | |In clinical trials |
| − | |align = "center"|'''16.7'''
| + | |
| | |- | | |- |
| − | |align = "center"|'''Mexico''' | + | |COL-177 |
| − | |align = "center"|9 | + | |Onset Dermatologics |
| − | |align = "center"|2 | + | |Acne vulgaris |
| − | |align = "center"|11 | + | |In clinical trials |
| − | |align = "center"|'''18.1'''
| + | |
| | |- | | |- |
| − | |align = "center"|'''India''' | + | |Duac Topical Gel low-dose clindamycin/benzoyl peroxide gel |
| − | |align = "center"|6 | + | |Stiefel, a GSK company |
| − | |align = "center"|3 | + | |Acne vulgaris |
| − | |align = "center"|9 | + | |Application submitted |
| − | |align = "center"|'''33.3'''
| + | |
| | |- | | |- |
| − | |} | + | |E-0301 |
| − | Source: Kalorama Information
| + | |Elorac |
| − | | + | |Acne |
| − | ===Rx to OTC Switches===
| + | |Phase III |
| − | {|align="center"
| + | |
| − | |[[Image:Rx to OTC Switches market1.png|left|thumb|800px|Source:Kalorama Information]][[Image:Market Segmentation (2008).png|right|thumb|800px|Source:Kalorama Information]] | + | |
| | |- | | |- |
| − | |} | + | |IDP 107 |
| − | *CAGR for 2008-2013 is '''9.4%'''
| + | |Valeant Pharmaceuticals |
| − | | + | |Acne vulgaris |
| − | ===Major OTC Players===
| + | |Phase II |
| − | [[Image:OTC Players1.png|center|thumb|800px|Source:Company Websites]]
| + | |
| − | <br>
| + | |
| − | | + | |
| − | ===Sales Data for selected Rx to OTC Switches, USA===
| + | |
| − | {|border="2" cellspacing="0" cellpadding="4" width="100%"
| + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''S.No''' | + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Switched Drug'''
| + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Manufacturer/Marketer'''
| + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Drug Category'''
| + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Switch Year'''
| + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''First 12-Month Sales (in $M)'''
| + | |
| | |- | | |- |
| − | |align = "center"|1 | + | |Isotretinoin |
| − | |align = "center"|Alli | + | |Cipher Pharmaceuticals |
| − | |align = "center"|Glaxo SmithKline Consumer Healthcare | + | |Acne |
| − | |align = "center"|Weight Loss Aid | + | |Phase III |
| − | |align = "center"|2007
| + | |
| − | |align = "center" bgcolor = "#DBE5F1"|80
| + | |
| | |- | | |- |
| − | |align = "center"|2 | + | |JNJ-10229570 |
| − | |align = "center"|MiraLax
| + | |Johnson & Johnson |
| − | |align = "center"|Schering-Plough Healthcare
| + | |Acne vulgaris |
| − | |align = "center"|Gastrointestinal | + | |Phase II |
| − | |align = "center"|2006 | + | |
| − | |align = "center" bgcolor = "#DBE5F1"|40 | + | |
| | |- | | |- |
| − | |align = "center"|3 | + | |Metvixia |
| − | |align = "center"|Prilosec OTC | + | |PhotoCure methyl aminolevulinate |
| − | |align = "center"|Procter & Gamble | + | |Acne |
| − | |align = "center"|Gastrointestinal | + | |Phase II |
| − | |align = "center"|2003
| + | |
| − | |align = "center" bgcolor = "#DBE5F1"|130
| + | |
| | |- | | |- |
| − | |align = "center"|4 | + | |NB-003 (nanoemulsion) |
| − | |align = "center"|Claritin
| + | |NanoBio |
| − | |align = "center"|Schering-Plough Healthcare
| + | |Acne |
| − | |align = "center"|Allergy | + | |Phase I |
| − | |align = "center"|2002 | + | |
| − | |align = "center" bgcolor = "#DBE5F1"|380 | + | |
| | |- | | |- |
| − | |align = "center"|5 | + | |NVC-422 |
| − | |align = "center"|Nicoderm CQ | + | |Galderma |
| − | |align = "center"|Glaxo SmithKline Consumer Healthcare | + | |Acne |
| − | |align = "center"|Smoking Cessation | + | |Phase I completed |
| − | |align = "center"|1996
| + | |
| − | |align = "center" bgcolor = "#DBE5F1"|160
| + | |
| | |- | | |- |
| − | |align = "center"|6 | + | |Tazarotene foam (retinoid foam) |
| − | |align = "center"|Nicorette | + | |Stiefel, a GSK company |
| − | |align = "center"|Glaxo SmithKline Consumer Healthcare | + | |Acne vulgaris |
| − | |align = "center"|Smoking Cessation | + | |Phase III |
| − | |align = "center"|1996
| + | |
| − | |align = "center" bgcolor = "#DBE5F1"|195
| + | |
| | |- | | |- |
| − | |align = "center"|7 | + | |Tretinoin topical |
| − | |align = "center"|Rogaine | + | |Phosphagenics |
| − | |align = "center"|McNeil Consumer Healthcare | + | |Acne |
| − | |align = "center"|Hair Loss | + | |Phase I |
| − | |align = "center"|1996
| + | |
| − | |align = "center" bgcolor = "#DBE5F1"|180
| + | |
| | |- | | |- |
| − | |align = "center"|8 | + | |WC-3018 |
| − | |align = "center"|Pepcid AC
| + | |Warner Chilcott |
| − | |align = "center"|Johnson & Johnson- Merck Consumer Pharmaceuticals Co.
| + | |Acne |
| − | |align = "center"|Gastrointestinal | + | |Phase II |
| − | |align = "center"|1995 | + | |
| − | |align = "center" bgcolor = "#DBE5F1"|200 | + | |
| | |- | | |- |
| − | |align = "center"|9 | + | |WC-3035 |
| − | |align = "center"|Zantac 75 | + | |Warner Chilcott |
| − | |align = "center"|Boehringer Ingelheim Consumer Healthcare Products | + | |Acne |
| − | |align = "center"|Gastrointestinal | + | |Phase I |
| − | |align = "center"|1995
| + | |
| − | |align = "center" bgcolor = "#DBE5F1"|140
| + | |
| | |- | | |- |
| − | |align = "center"|10 | + | |}<br clear="all"> |
| − | |align = "center"|Aleve | + | |
| − | |align = "center"|Bayer Consumer Care
| + | {|border="0" cellspacing="0" cellpadding = "0" width="100%" |
| − | |align = "center"|Analgesic
| + | |<font color="#282828"> Source: [http://www.phrma.org/sites/default/files/pdf/skindiseases2011.pdf Pharmaceutical Research and Manufacturers of America, 2011] |
| − | |align = "center"|1994
| + | |
| − | |align = "center" bgcolor = "#DBE5F1"|110 | + | |
| − | |-
| + | |
| − | |}
| + | |
| − | Source:Company Websites, Press Releases and Journals | + | |
| | | | |
| − | ===Factors Affecting Rx to OTC Switch===
| |
| − | [[Image:Factors.png|center|thumb|800px]]
| |
| | | | |
| − | ==Trends in Rx to OTC Switches== | + | == '''USA market study''' == |
| − | ===Rx-to-OTC Switches Since 2000===
| + | |
| − | {|border="2" cellspacing="0" cellpadding="4" width="100%"
| + | ===Historic Sales=== |
| − | |align = "center" bgcolor = "#8DB4E3"|'''S.No'''
| + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Ingredient'''
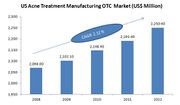
| + | * The Acne Treatment Manufacturing OTC industry has maintained strong growth during the past five years. As per IBIS World estimates from 2007 to 2012, revenue has grown steady, and it is expected to grow at an average annual rate of 2.4% to $2.3 billion. |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Product Category'''
| + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Date of OTC Approval'''
| + | * Because acne-prone individuals consider treatment products a necessary expense, revenue continued to rise during the recession, though growth did slow as consumers switched to more inexpensive products. |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Product Examples'''
| + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Company'''
| + | * Increasing prevalence of adult-onset and persistent acne conditions has given rise to new, targeted products and an expanded consumer base, another factor that has aided revenue growth during the period. |
| − | |-
| + | |
| − | |align = "center"|1
| + | * In 2012, industry revenue is expected to maintain its growth, gaining 2.7% during the year, due to modest improvements in per capita disposable income. |
| − | |align = "center"|ibuprofen (NDA)
| + | |
| − | |align = "center"|migraine
| + | |
| − | |align = "center"|25/02/2000
| + | [[Image:USACNE2012.jpg|center|thumb|center|632px * 341px|Source:IBIS World,[http://www.ibisworld.com/industry/acne-treatment-manufacturing-otc.html]]] |
| − | |align = "center"|Motrin Migraine Pain
| + | |
| − | |align = "center"|McNeil Consumer Healthcare
| + | |
| − | |-
| + | |
| − | |align = "center"|2
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|docosanol (NDA)
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|cold sore/fever blister
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|25/07/2000
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|Abreva Cream
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|Avanir Pharmaceuticals
| + | |
| − | |-
| + | |
| − | |align = "center" rowspan = "2"|3
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|famotidine, calcium carbonate,
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|heartburn,
| + | |
| − | |align = "center" bgcolor = "#B8CCE4" rowspan = "2"|17/10/2000
| + | |
| − | |align = "center" bgcolor = "#B8CCE4" rowspan = "2"|Pepcid Complete
| + | |
| − | |align = "center" bgcolor = "#B8CCE4" rowspan = "2"|J&J/Merck
| + | |
| − | |-
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|magnesium hydroxide (NDA)
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|acid indigestion
| + | |
| − | |-
| + | |
| − | |align = "center"|4
| + | |
| − | |align = "center"|butenafine hydrochloride (NDA)
| + | |
| − | |align = "center"|athlete's foot, jock itch, ringworm
| + | |
| − | |align = "center"|7/12/2001
| + | |
| − | |align = "center"|Lotrimin Ultra
| + | |
| − | |align = "center"|Schering-Plough
| + | |
| − | |-
| + | |
| − | |align = "center" rowspan = "2"|5
| + | |
| − | |align = "center"|ibuprofen, pseudoephedrine HCl,
| + | |
| − | |align = "center" rowspan = "2"|analgesic/decongestant
| + | |
| − | |align = "center" rowspan = "2"|18/04/2002
| + | |
| − | |align = "center" rowspan = "2"|Children’s Advil Cold
| + | |
| − | |align = "center" rowspan = "2"|Wyeth
| + | |
| − | |-
| + | |
| − | |align = "center"|suspension for pediatric use (NDA)
| + | |
| − | |-
| + | |
| − | |align = "center"|6
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|guaifenesin extended-release tablet (NDA)
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|expectorant
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|12/7/2002
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|Mucinex
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|Adams Respiratory Therapeutics
| + | |
| − | |-
| + | |
| − | |align = "center"|7
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|nicotine polacrilex troche/lozenge (NDA)
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|smoking cessation
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|31/10/2002
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|Commit
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|GlaxoSmithKline
| + | |
| − | |-
| + | |
| − | |align = "center"|8
| + | |
| − | |align = "center"|loratadine (NDA)
| + | |
| − | |align = "center"|antihistamine
| + | |
| − | |align = "center"|27/11/2002
| + | |
| − | |align = "center"|Claritin Tablets, Claritin RediTabs, Claritin Syrup
| + | |
| − | |align = "center"|Schering-Plough
| + | |
| − | |-
| + | |
| − | |align = "center" rowspan = "2"|9
| + | |
| − | |align = "center" rowspan = "2"|loratadine, pseudoephededrine sulfate (NDA)
| + | |
| − | |align = "center"|antihistamine/
| + | |
| − | |align = "center" rowspan = "2"|27/11/2002
| + | |
| − | |align = "center" rowspan = "2"|Claritin-D 12 Hour Extended Release Tablets,
| + | |
| − | |align = "center" rowspan = "2"|Schering-Plough
| + | |
| − | |-
| + | |
| − | |align = "center"|decongestant
| + | |
| − | |-
| + | |
| − | |align = "center"|10
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|omeprazole magnesium
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|acid reducer to treat frequent heartburn
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|20/06/2003
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|Prilosec OTC
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|Procter & Gamble | + | |
| − | |- | + | |
| − | |align = "center" rowspan = "2"|11
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|loratadine (NDA) | + | |
| − | |align = "center" bgcolor = "#B8CCE4"|hives relief
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|15/11/2003
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|Claritin hives relief
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|Schering-Plough
| + | |
| − | |-
| + | |
| − | |align = "center"|diphenhydramine citrate & ibuprofen (NDA); diphenhydramine HCl & ibuprofen potassium (NDA)
| + | |
| − | |align = "center"|analgesic sleep-aid
| + | |
| − | |align = "center"|21/12/2005
| + | |
| − | |align = "center"|Advil PM
| + | |
| − | |align = "center"|Wyeth
| + | |
| − | |-
| + | |
| − | |align = "center"|12
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|ecamsule (combined with avobenzone and octocrylene (NDA)
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|sunscreen
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|21/07/2006
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|Anthelios SX
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|L’Oreal
| + | |
| − | |-
| + | |
| − | |align = "center" rowspan = "2"|13
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|levonorgestrel (NDA)
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|contraceptive
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|24/08/2006
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|Plan B
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|Duramed
| + | |
| − | |-
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|polyethylene glycol 3350 (NDA)
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|laxative
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|6/10/2006
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|MiraLAX
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|Schering-Plough
| + | |
| − | |-
| + | |
| − | |align = "center"|14
| + | |
| − | |align = "center"|ketotifen (NDA)
| + | |
| − | |align = "center"|antihistamine eye drops
| + | |
| − | |align = "center"|19/10/2006
| + | |
| − | |align = "center"|Zaditor
| + | |
| − | |align = "center"|Novartis
| + | |
| − | |-
| + | |
| − | |align = "center" rowspan = "2"|15
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|orlistat (NDA)
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|weight loss aid
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|7/2/2007
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|alli
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|GlaxoSmithKline
| + | |
| − | |-
| + | |
| − | |align = "center" bgcolor = "#B8CCE4" rowspan = "2"|cetirizine HCl & pseudoephedrine HCl (NDA)
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|antihistamine/
| + | |
| − | |align = "center" bgcolor = "#B8CCE4" rowspan = "2"|9/11/2007
| + | |
| − | |align = "center" bgcolor = "#B8CCE4" rowspan = "2"|Zyrtec-D
| + | |
| − | |align = "center" bgcolor = "#B8CCE4" rowspan = "2"|McNeil
| + | |
| − | |-
| + | |
| − | |align = "center"|16
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|decongestant
| + | |
| − | |-
| + | |
| − | |align = "center" rowspan = "2"|17
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|cetirizine HCl (NDA)
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|antihistamine, hives relief
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|16/11/2007
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|Zyrtec
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|McNeil
| + | |
| − | |-
| + | |
| − | |align = "center" bgcolor = "#CCC0DA" rowspan = "2"|lansoprazole (NDA)
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|acid reducer to treat
| + | |
| − | |align = "center" bgcolor = "#CCC0DA" rowspan = "2"|18/05/2009
| + | |
| − | |align = "center" bgcolor = "#CCC0DA" rowspan = "2"|Prevacid 24 HR
| + | |
| − | |align = "center" bgcolor = "#CCC0DA" rowspan = "2"|Novartis
| + | |
| − | |-
| + | |
| − | |align = "center"|18
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|frequent heartburn
| + | |
| − | |-
| + | |
| − | |align = "center" rowspan = "2"|19
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|levonorgestrel (NDA)
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|contraceptive
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|10/7/2009
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|Plan B One Step
| + | |
| − | |align = "center" bgcolor = "#CCC0DA"|Duramed
| + | |
| − | |-
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|omeprazole and sodium
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|acid reducer to treat
| + | |
| − | |align = "center" bgcolor = "#B8CCE4" rowspan = "2"|1/12/2009
| + | |
| − | |align = "center" bgcolor = "#B8CCE4" rowspan = "2"|Zegerid OTC
| + | |
| − | |align = "center" bgcolor = "#B8CCE4" rowspan = "2"|Schering-Plough
| + | |
| − | |-
| + | |
| − | |align = "center"|20
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|bicarbonate (NDA)
| + | |
| − | |align = "center" bgcolor = "#B8CCE4"|frequent heartburn
| + | |
| − | |-
| + | |
| − | |}
| + | |
| | | | |
| − | * Blue highlighted drugs are patent-protected<br>
| + | ===Industry Forecast=== |
| − | * Purple highlighted drugs are under FDA exclusivity period
| + | |
| | | | |
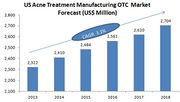
| − | ===Rx to OTC Switches before patent expiry=== | + | * From 2012 to 2017, industry revenue is expected to go up an annualized rate of 3.1% to $2.7 billion from current level of $2.3 billion. |
| − | {|border="2" cellspacing="0" cellpadding="4" width="100%" | + | * Improving economic conditions is likely to drive the market, as greater disposable income growth allows consumers to spend on higher-priced over-the-counter (OTC) items. |
| − | |align = "center" bgcolor = "#8DB4E3"|'''S.No''' | + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Product Examples''' | + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Company''' | + | [[Image:US2018 acne.jpg|center|thumb|center|632px * 341px|Source:IBIS World,[http://www.ibisworld.com/industry/acne-treatment-manufacturing-otc.html]]] |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Date of OTC Approval''' | + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Patent Expiry''' | + | ===Major Players in Acne Space=== |
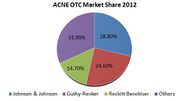
| − | |align = "center" bgcolor = "#8DB4E3"|'''FDA Exclusivity''' | + | * As per IBIS World estimates the top three industry players (Johnson & Johnson, Guthy-Renker and Reckitt-Benckiser) hold a combined market share of about 68.1%, in 2012. The rest of the market is characterized by a large number of small and medium-size businesses. |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Patent Number''' | + | |
| | + | * J&J (Neutrogena, Clean & Clear, Aveeno, Ambi) commanded over 28.8% of anti-acne preparation sales in 2012. J&J’s closest rival was Guthy Renker,(Proactiv Solutions) which had a 24.6% share followed by Reckitt Benckiser at 14.7%. |
| | + | |
| | + | [[Image:US Acne Share.jpg|US Acne Share.jpg|thumb|center|632px * 341px|Source:IBIS World,[http://www.ibisworld.com/industry/acne-treatment-manufacturing-otc.html]]] |
| | + | |
| | + | ===Consumer Target Group & Product Categorization=== |
| | + | |
| | + | '''Consumer Target Group''' |
| | + | |
| | + | Here we have divided entire population into various categories. This categories are defined as follows: |
| | + | |
| | + | # Teenager/Young Adult : Age 13 to 20 years |
| | + | # Adult : Age 25 and Above |
| | + | # General population |
| | + | |
| | + | '''Product Categorization''' |
| | + | |
| | + | Here, we had tried to categorize the the different products under following 3 categories. |
| | + | |
| | + | # Devices |
| | + | # Kits |
| | + | # Moisturizers/Lotion/cream |
| | + | |
| | + | The below information is regarding the target group and categories are obtained from company websites and review published on various online pharmacy sites. |
| | + | |
| | + | {|border="2" cellspacing="0" width="100%" |
| | + | |align = "center" bgcolor = "#FFCC99"|'''Company''' |
| | + | |align = "center" bgcolor = "#FFCC99"|'''Brand''' |
| | + | |align = "center" bgcolor = "#FFCC99"|'''Category''' |
| | + | |align = "center" bgcolor = "#FFCC99"|'''Target''' |
| | + | |align = "center" bgcolor = "#FFCC99"|'''Company''' |
| | + | |align = "center" bgcolor = "#FFCC99"|'''Brand''' |
| | + | |align = "center" bgcolor = "#FFCC99"|'''Category''' |
| | + | |align = "center" bgcolor = "#FFCC99"|'''Target''' |
| | |- | | |- |
| − | |align = "center"|1 | + | |Johnson & Johnson |
| − | |align = "center" |Abreva Cream | + | |<font color="#0000FF"><u>[http://www.ambiskincare.com/ Ambi]</u></font> |
| − | |align = "center" |Avanir Pharmaceuticals | + | |Moistureriser/Lotion/Cream |
| − | |align = "center" |25/07/2000 | + | |General Population |
| − | |align = "center" |28/04/2014
| + | |Mentholatum Co. |
| − | |align = "center" |-- | + | |<font color="#0000FF"><u>[http://www.oxymom.com/?event=prod.bodywash OXY Body Wash 2% Salicylic Acid Acne Treatment, Island Wave]</u></font> |
| − | |align = "center" |4874794 | + | |Moistureriser/Lotion/Cream |
| | + | |Teen and Adult |
| | |- | | |- |
| − | |align = "center"|2 | + | |Johnson & Johnson |
| − | |align = "center" |Pepcid Complete | + | |<font color="#0000FF"><u>[http://www.aveeno.com/productDetail.do?prodid=3811 Aveeno Clear Complexion]</u></font> |
| − | |align = "center" |J&J/Merck | + | |Moistureriser/Lotion/Cream |
| − | |align = "center" |17/10/2000 | + | |General Population |
| − | |align = "center" |15/10/2000
| + | |Mentholatum Co. |
| − | |align = "center" |-- | + | |<font color="#0000FF"><u>[http://www.oxymom.com/?event=prod.bodywash OXY Face Wash Acne-Fighting Formula]</u></font> |
| − | |align = "center" |4283408 | + | |Moistureriser/Lotion/Cream |
| | + | |Teen |
| | |- | | |- |
| − | |align = "center"|3 | + | |Johnson & Johnson |
| − | |align = "center" |Mucinex | + | |<font color="#0000FF"><u>[http://www.cleanandclear.com/ Clean & Clear Advantage Acne Cleanser]</u></font> |
| − | |align = "center" |Adams Respiratory Therapeutics | + | |Kits and Moistureriser/Lotion/Cream |
| − | |align = "center" |12/7/2002 | + | |Adult |
| − | |align = "center" |28/04/2020
| + | |Mentholatum Co. |
| − | |align = "center" |-- | + | |<font color="#0000FF"><u>[http://www.oxymom.com/?event=prod.bodywash Oxy chill factor Face Scrub Acne treatment]</u></font> |
| − | |align = "center" |6372252 | + | |Moistureriser/Lotion/Cream |
| | + | |Teen and Adult |
| | |- | | |- |
| − | |align = "center"|4 | + | |Johnson & Johnson |
| − | |align = "center" |Commit | + | |<font color="#0000FF"><u>[http://www.neutrogena.com/ Neutrogena Acne Stress Control]</u></font> |
| − | |align = "center" |GlaxoSmithKline | + | |Moistureriser/Lotion/Cream |
| − | |align = "center" |31/10/2002 | + | |Teen and Adult |
| − | |align = "center" |21/08/2010 | + | |Mentholatum Co. |
| − | |align = "center" |-- | + | |<font color="#0000FF"><u>[http://www.phisoderm.com/anti-blemish_body.html Phisoderm Anti-Blemish Body Wash]</u></font> |
| − | |align = "center" |5110605
| + | |Moistureriser/Lotion/Cream |
| | + | |General Population |
| | |- | | |- |
| − | |align = "center"|5 | + | |Johnson & Johnson |
| − | |align = "center" |Prilosec OTC | + | |<font color="#0000FF"><u>[http://www.neutrogena.com/ Neutrogena Advanced Solutions]</u></font> |
| − | |align = "center" |Procter & Gamble | + | |Moistureriser/Lotion/Cream |
| − | |align = "center" |20/06/2003 | + | |Teen and Adult |
| − | |align = "center" |15/11/2019 | + | |Mentholatum Co. |
| − | |align = "center" |-- | + | |<font color="#0000FF"><u>[http://www.phisoderm.com/anti-blemish_pads.html Phisoderm Anti-Blemish Cleansing Pads]</u></font> |
| − | |align = "center" |5690960, 5753265, 5817338, 5900424, 6403616, 6428810
| + | |Moistureriser/Lotion/Cream |
| | + | |Teen and Adult |
| | |- | | |- |
| − | |align = "center"|6 | + | |Johnson & Johnson |
| − | |align = "center" |Claritin hives relief | + | |<font color="#0000FF"><u>[http://www.neutrogena.com/ Neutrogena Clear Pore]</u></font> |
| − | |align = "center" |Schering-Plough | + | |Moistureriser/Lotion/Cream |
| − | |align = "center" |15/11/2003 | + | |Teen and Adult |
| − | |align = "center" |19/06/2002
| + | |Kao Co., Ltd. |
| − | |align = "center" |-- | + | |<font color="#0000FF"><u>[http://www.biore.com/usa/products/productInfo.asp?productId=2 Biore Blemish Fighting Ice Cleanser]</u></font> |
| − | |align = "center" |4282233 | + | |Kits and Moistureriser/Lotion/Cream |
| | + | |Teen and Adult |
| | |- | | |- |
| − | |align = "center"|7 | + | |Johnson & Johnson |
| − | |align = "center" |Anthelios SX | + | |<font color="#0000FF"><u>[http://www.neutrogena.com/ Neutrogena Healthy Skin]</u></font> |
| − | |align = "center" |L’Oreal | + | |Moistureriser/Lotion/Cream |
| − | |align = "center" |21/07/2006 | + | |Teen and Adult |
| − | |align = "center" |24/12/2013
| + | |Kao Co., Ltd. |
| − | |align = "center" |-- | + | |<font color="#0000FF"><u>[http://www.biore.com/usa/products/productInfo.asp?productId=3 Biore Warming Anti-Blackhead Cream Cleanser]</u></font> |
| − | |align = "center" |5587150 | + | |Moistureriser/Lotion/Cream |
| | + | |Adult |
| | |- | | |- |
| − | |align = "center"|8 | + | |Johnson & Johnson |
| − | |align = "center" bgcolor = "#CCC0DA"|Plan B | + | |<font color="#0000FF"><u>[http://www.neutrogena.com/ Neutrogena Oil Free Acne Wash]</u></font> |
| − | |align = "center" bgcolor = "#CCC0DA"|Duramed | + | |Moistureriser/Lotion/Cream |
| − | |align = "center" bgcolor = "#CCC0DA"|24/08/2006
| + | |Teen and Adult |
| − | |align = "center" bgcolor = "#CCC0DA"|-- | + | |Alberto-Culver Co. |
| − | |align = "center" bgcolor = "#CCC0DA"|24/08/2009 | + | |<font color="#0000FF"><u>[http://www.stives.com/skin-care-products/facial-invigorating-scrub.cfm St Ives Apricot Face Wash]</u></font> |
| − | |align = "center" bgcolor = "#CCC0DA"|<nowiki>-</nowiki>-
| + | |Moistureriser/Lotion/Cream |
| | + | |Teen and Adult |
| | |- | | |- |
| − | |align = "center"|9 | + | |Johnson & Johnson |
| − | |align = "center" bgcolor = "#CCC0DA"|MiraLAX | + | |<font color="#0000FF"><u>[http://www.neutrogena.com/ Neutrogena On the Spot]</u></font> |
| − | |align = "center" bgcolor = "#CCC0DA"|Schering-Plough | + | |Moistureriser/Lotion/Cream |
| − | |align = "center" bgcolor = "#CCC0DA"|6/10/2006
| + | |Teen and Adult |
| − | |align = "center" bgcolor = "#CCC0DA"|-- | + | |Blistex, Inc. |
| − | |align = "center" bgcolor = "#CCC0DA"|6/10/2009 | + | |<font color="#0000FF"><u>[http://www.stridex.com/ Stridex]</u></font> |
| − | |align = "center" bgcolor = "#CCC0DA"|<nowiki>-</nowiki>-
| + | |Moistureriser/Lotion/Cream |
| | + | |Teen and Adult |
| | |- | | |- |
| − | |align = "center"|10 | + | |Johnson & Johnson |
| − | |align = "center" |alli | + | |<font color="#0000FF"><u>[http://www.neutrogena.com/ Neutrogena Men Skin Clearing Face Wash]</u></font> |
| − | |align = "center" |GlaxoSmithKline | + | |Moistureriser/Lotion/Cream |
| − | |align = "center" |7/2/2007 | + | |Teen and Adult |
| − | |align = "center" |6/1/2018
| + | |The Procter & Gamble Co. |
| − | |align = "center" |7/2/2010 | + | |<font color="#0000FF"><u>[http://www.noxzema.com/ Noxzema]</u></font> |
| − | |align = "center" |6004996 | + | |Kits and Moistureriser/Lotion/Cream |
| | + | |Adult |
| | |- | | |- |
| − | |align = "center"|11 | + | |Johnson & Johnson |
| − | |align = "center" |Zyrtec-D | + | |<font color="#0000FF"><u>[http://www.neutrogena.com/ Neutrogena Rapid Clear]</u></font> |
| − | |align = "center" |McNeil | + | |Moistureriser/Lotion/Cream |
| − | |align = "center" |9/11/2007 | + | |Teen and Adult |
| − | |align = "center" |10/6/2022
| + | |The Procter & Gamble Co. |
| − | |align = "center" |<nowiki>-</nowiki>-
| + | |<font color="#0000FF"><u>[http://www.olay.com/boutique/dailyfacials/ Olay Daily Facials Clarity ]</u></font> |
| − | |align = "center" |6469009, 6489329, 7014867, 7226614 | + | |Moistureriser/Lotion/Cream |
| | + | |Teen and Adult |
| | |- | | |- |
| − | |align = "center"|12 | + | |Reckitt Benckiser PLC |
| − | |align = "center" |Zyrtec | + | |<font color="#0000FF"><u>[http://www.clearasil.us/creams/creams_vanishing.shtml Clearasil Daily Acne Control Vanishing Acne Treatment Cream]</u></font> |
| − | |align = "center" |McNeil | + | |Moistureriser/Lotion/Cream |
| − | |align = "center" |16/11/2007 | + | |Teen and Adult |
| − | |align = "center" |2/7/2018
| + | |Stiefel Laboratories, Inc. |
| − | |align = "center" |<nowiki>-</nowiki>-
| + | |<font color="#0000FF"><u>[http://www.stiefel.com/products/ Stiefel Panoxyl]</u></font> |
| − | |align = "center" |6455533 | + | |Kits and Moistureriser/Lotion/Cream |
| | + | |Teen and Adult |
| | |- | | |- |
| − | |align = "center"|13 | + | |Reckitt Benckiser PLC |
| − | |align = "center" bgcolor = "#CCC0DA"|Prevacid 24 HR | + | |<font color="#0000FF"><u>[http://www.clearasil.us/creams/creams_adult.shtml Clearasil Acne Control Adult Acne Treatment Cream]</u></font> |
| − | |align = "center" bgcolor = "#CCC0DA"|Novartis | + | |Moistureriser/Lotion/Cream |
| − | |align = "center" bgcolor = "#CCC0DA"|18/05/2009
| + | |Teen and Adult |
| − | |align = "center" bgcolor = "#CCC0DA"|-- | + | |Bristol-Myers Squibb |
| − | |align = "center" bgcolor = "#CCC0DA"|18/05/2012 | + | |<font color="#0000FF"><u>[http://www.pgbeautyscience.com/ Sea Breeze]</u></font> |
| − | |align = "center" bgcolor = "#CCC0DA"|<nowiki>-</nowiki>-
| + | |Moistureriser/Lotion/Cream |
| | + | |Teen and Adult |
| | |- | | |- |
| − | |align = "center"|14 | + | |University Medical |
| − | |align = "center" bgcolor = "#CCC0DA"|Plan B One Step | + | |<font color="#0000FF"><u>[http://www.acnefree.com/af University Medical]</u></font> |
| − | |align = "center" bgcolor = "#CCC0DA"|Duramed | + | |Kits and Moistureriser/Lotion/Cream |
| − | |align = "center" bgcolor = "#CCC0DA"|10/7/2009
| + | |Teen and Adult |
| − | |align = "center" bgcolor = "#CCC0DA"|-- | + | |Bristol-Myers Squibb |
| − | |align = "center" bgcolor = "#CCC0DA"|10/7/2012 | + | |<font color="#0000FF"><u>[http://www.pgbeautyscience.com/ Sea Breeze Naturals Gentle Cream Cleanser]</u></font> |
| − | |align = "center" bgcolor = "#CCC0DA"|<nowiki>-</nowiki>-
| + | |Kits and Moistureriser/Lotion/Cream |
| | + | |Teen and Adult |
| | |- | | |- |
| − | |align = "center"|15 | + | |University Medical |
| − | |align = "center" |Zegerid OTC | + | |<font color="#0000FF"><u>[http://www.acnefree.com/af University Medical AcneFree Clear Skin System]</u></font> |
| − | |align = "center" |Schering-Plough | + | |Kits and Moistureriser/Lotion/Cream |
| − | |align = "center" |1/12/2009
| + | |Adult |
| − | |align = "center" |15/07/2016 | + | |Nature<nowiki>’</nowiki>s Cure, Inc. |
| − | |align = "center" |<nowiki>-</nowiki>- | + | |<font color="#0000FF"><u>[http://www.naturescure.com/ Nature<nowiki>’</nowiki>s Cure]</u></font> |
| − | |align = "center" |6489346, 6645988, 6699885, 7399772 | + | |Moistureriser/Lotion/Cream |
| | + | |Teen and Adult |
| | |- | | |- |
| | |} | | |} |
| | | | |
| − | * Purple highlighted drugs are under FDA exclusivity period
| + | === OTC Products === |
| | | | |
| − | ===Trend Analysis=== | + | This report covers the product available in mass distribution channel only. |
| − | #11 out of 22 recent drugs ('''50%''') which switched from Rx to OTC were '''patent protected'''
| + | |
| − | #4 out of 22 recent drugs ('''18.2%''') which switched from Rx to OTC were under '''FDA exclusivity period'''
| + | === Product Dashboard === |
| − | #13 out of 22 recent drugs ('''59%''') were switched from Rx to OTC before their patent or FDA exclusivity expiry. 2 drugs switched after the expiry of patents
| + | |
| − | #6 out of these 13 drugs made a switch more than 10 years before expiry
| + | [http://client.dolcera.com/dashboard/dashboard.html?workfile_id=513 Link to the Product Dashboard] |
| − | #5 out of these 13 drugs made a switch between 3 to 10 years before expiry
| + | |
| − | <br>
| + | Snapshot of the dashboard |
| | + | |
| | + | [[Image:Acne product Dashboard.jpg|850px]] |
| | + | |
| | + | |
| | + | =='''Japan Market Study'''== |
| | + | |
| | + | ===Market Facts=== |
| | + | |
| | + | * Acne treatments most dynamic with 20% current value growth in 2007 |
| | + | |
| | + | * Acne treatments were the best performing product area in 2007, achieving 20% current value growth over the previous year. This was chiefly due to the re- launch of leading brand Mentholatum Acnes from Rohto Pharmaceutical Co Ltd in 2006. The brand was re -launched with a more effective formulation and grew share dynamically in 2007 as a result. |
| | + | |
| | + | * Acne treatments are also expected to see strong growth during the forecast period, with constant value growth of 42%. Growth will be supported by further new product development. The growing number of busy and affluent young workers suffering from acne will make this product area highly attractive to players during the forecast period, with these consumers generally willing to pay more for products that are convenient and enhance their appearance. New launches are therefore expected to focus on swiftly absorbed formulations that also claim to improve skin condition. |
| | + | |
| | + | === Historic Sales === |
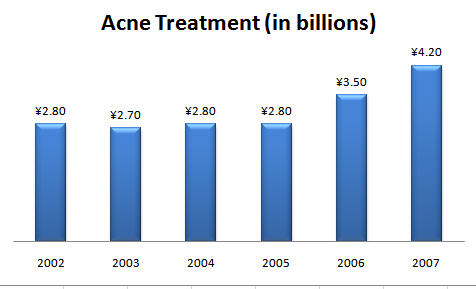
| | + | Following figure shows the sales of acne treatment product during the period 2004 to 2007. |
| | + | |
| | + | [[Image:Acne Treatment.jpg|700px|thumb|center|Source : Euromonitor International]] |
| | | | |
| − | ===Potential Drugs for Rx-to-OTC Switch=== | + | ===Brands and their Sales=== |
| − | {|border="2" cellspacing="0" cellpadding="4" width="100%" | + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''S.No''' | + | * The below table shows the names of various brands under acne care and their respective '''sales percentage''' for the period between 2004 to 2007. |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Drug'''
| + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Patent Expiry Date'''
| + | |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Expected Switch Year'''
| + | {|border="2" cellspacing="0" cellpadding="4" width="57%" |
| − | |align = "center" bgcolor = "#8DB4E3"|'''US Patent Numbers'''
| + | |align = "center" colspan = "5"|Acne Treatments Brand Shares by Value 2004-2007 |
| | |- | | |- |
| − | |align = "center"|1 | + | |bgcolor = "#FDE9D9"|Brand |
| − | |align = "center"|Allegra | + | |align = "right" bgcolor = "#FDE9D9"|2004 |
| − | |align = "center"|14/3/2017 | + | |align = "right" bgcolor = "#FDE9D9"|2005 |
| − | |align = "center"|2012 | + | |align = "right" bgcolor = "#FDE9D9"|2006 |
| − | |align = "center"|5578610, 6037353, 6187791, 6399632, 7138524 | + | |align = "right" bgcolor = "#FDE9D9"|2007 |
| | |- | | |- |
| − | |align = "center"|2 | + | |Mentholatum Acnes(Oxy) |
| − | |align = "center"|Clarinex | + | |align = "right"|13.4 |
| − | |align = "center"|1/12/2018 | + | |align = "right"|13.4 |
| − | |align = "center"|<nowiki>></nowiki>2013 | + | |align = "right"|14.3 |
| − | |align = "center"| 6514520, 7211582, 7214683, 7214684 | + | |align = "right"|19.1 |
| | |- | | |- |
| − | |align = "center"|3 | + | |Clearasil Acne |
| − | |align = "center"|Crestor | + | |align = "right"|19.5 |
| − | |align = "center"|17/6/2022 | + | |align = "right"|22 |
| − | |align = "center"|<nowiki>></nowiki>2014 | + | |align = "right"|17.5 |
| − | |align = "center"|6858618, 6316460, 7030152, RE37314 | + | |align = "right"|15.5 |
| | |- | | |- |
| − | |align = "center"|4 | + | |Pair Acne |
| − | |align = "center"|Lescol | + | |align = "right"|5.4 |
| − | |align = "center"|12/6/2012 | + | |align = "right"|5.4 |
| − | |align = "center"|<nowiki>></nowiki>2014 | + | |align = "right"|17.2 |
| − | |align = "center"|5356896, 5354772, | + | |align = "right"|14.6 |
| | |- | | |- |
| − | |align = "center"|5 | + | |Bif Night |
| − | |align = "center"|Lipitor | + | |align = "right"|13.5 |
| − | |align = "center"|8/1/2017 | + | |align = "right"|14.4 |
| − | |align = "center"|<nowiki>></nowiki>2014 | + | |align = "right"|14.3 |
| − | |align = "center"|5969156, 4681893, 5273995, 5686104, 5969156, 6126971, RE40667 | + | |align = "right"|12.4 |
| | |- | | |- |
| − | |align = "center"|6 | + | |Pimplit |
| − | |align = "center"|Pravachol | + | |align = "right"|9.4 |
| − | |align = "center"|22/10/2014 | + | |align = "right"|9.4 |
| − | |align = "center"|<nowiki>></nowiki>2014 | + | |align = "right"|8.6 |
| − | |align = "center"|5622985 | + | |align = "right"|7.6 |
| | |- | | |- |
| − | |align = "center"|7 | + | |Menturm Acne Lotion |
| − | |align = "center"|TriCor | + | |align = "right"|8.7 |
| − | |align = "center"|21/2/2023 | + | |align = "right"|8.6 |
| − | |align = "center"|<nowiki>></nowiki>2014 | + | |align = "right"|6.6 |
| − | |align = "center"|7276249, 5145684, 6277405, 6375986 , 6652881, 7037529, 7041319, 7320802 | + | |align = "right"|5.7 |
| | |- | | |- |
| − | |align = "center"|8 | + | |Skinlife |
| − | |align = "center"|Vytorin | + | |align = "right"|4 |
| − | |align = "center"|25/4/2017 | + | |align = "right"|4 |
| − | |align = "center"|<nowiki>></nowiki>2014 | + | |align = "right"|2.9 |
| − | |align = "center"|RE37721, 5846966, | + | |align = "right"|2.4 |
| | |- | | |- |
| − | |align = "center"|9 | + | |Pair Acne Cream W |
| − | |align = "center"|Zetia | + | | |
| − | |align = "center"|25/7/2022 | + | | |
| − | |align = "center"|<nowiki>></nowiki>2014 | + | |align = "right"|2.9 |
| − | |align = "center"|7030106, 5846966, 7612058, RE37721 | + | |align = "right"|2.4 |
| | |- | | |- |
| − | |align = "center"|10 | + | |Eva Youth |
| − | |align = "center"|AcipHex | + | | |
| − | |align = "center"|8/5/2013 | + | | |
| − | |align = "center"|2013 | + | | |
| − | |align = "center"|5045552 | + | |align = "right"|2.4 |
| | |- | | |- |
| − | |align = "center"|11 | + | |Acnepell |
| − | |align = "center"|Nexium | + | |align = "right"|3.3 |
| − | |align = "center"|25/11/2018 | + | |align = "right"|3.3 |
| − | |align = "center"|<nowiki>></nowiki>2014 | + | |align = "right"|2.3 |
| − | |align = "center"|7411070, 5690960, 5714504, 5877192, 5900424, 6147103, 6166213, 6191148, 6369085, 6428810, 6875872 | + | |align = "right"|2.1 |
| | |- | | |- |
| − | |align = "center"|12 | + | |Freshing Cream |
| − | |align = "center"|Protonix | + | |align = "right"|2.9 |
| − | |align = "center"|30/3/2025 | + | |align = "right"|2.9 |
| − | |align = "center"|2010 | + | |align = "right"|2 |
| − | |align = "center"|7553498, 4758579, 7544370, 7550153 | + | |align = "right"|1.9 |
| | |- | | |- |
| − | |align = "center"|13 | + | |Eskamel |
| − | |align = "center"|Zofran | + | |align = "right"|2.5 |
| − | |align = "center"|7/12/2026 | + | |align = "right"|2.5 |
| − | |align = "center"|<nowiki>></nowiki>2014 | + | |align = "right"|1.7 |
| − | |align = "center"|7544370, 4758579, 7550153, 7553498 | + | |align = "right"|1.7 |
| | |- | | |- |
| − | |align = "center"|14 | + | |Annsalbe |
| − | |align = "center"|Propecia | + | |align = "right"|1.8 |
| − | |align = "center"|5/11/2013 | + | |align = "right"|1.8 |
| − | |align = "center"|2011 | + | |align = "right"|1.1 |
| − | |align = "center"|5571817, 5547957, 5886184 | + | |align = "right"|1 |
| | |- | | |- |
| − | |align = "center"|15 | + | |Eva Youth |
| − | |align = "center"|Imitrex | + | |align = "right"|3.3 |
| − | |align = "center"|10/3/2014 | + | |align = "right"|3.3 |
| − | |align = "center"|<nowiki>></nowiki>2010 | + | |align = "right"|2.6 |
| − | |align = "center"|5554639, 5307953, 5705520 | + | | |
| | |- | | |- |
| − | |align = "center"|16 | + | |Private label |
| − | |align = "center"|Actonel | + | |align = "right"|2.2 |
| − | |align = "center"|10/12/2018 | + | |align = "right"|2.1 |
| − | |align = "center"|<nowiki>></nowiki>2014 | + | |align = "right"|1.4 |
| − | |align = "center"|6165513, 5583122, 6096342 | + | |align = "right"|1.2 |
| | |- | | |- |
| − | |align = "center"|17 | + | |Others |
| − | |align = "center"|Boniva | + | |align = "right"|10.1 |
| − | |align = "center"|2/9/2014
| + | |align = "right"|6.8 |
| − | |align = "center"|<nowiki>></nowiki>2014
| + | |align = "right"|4.6 |
| − | |align = "center"|5662918, 4927814
| + | |align = "right"|10 |
| − | |-
| + | |
| − | |align = "center"|18
| + | |
| − | |align = "center"|Fosamax
| + | |
| − | |align = "center"| 17/1/2019
| + | |
| − | |align = "center"|<nowiki>></nowiki>2014
| + | |
| − | |align = "center"|6225294, 5462932, 5994329, 6015801
| + | |
| − | |-
| + | |
| − | |align = "center"|19
| + | |
| − | |align = "center"|Evista
| + | |
| − | |align = "center"|10/3/2017
| + | |
| − | |align = "center"|<nowiki>></nowiki>2014
| + | |
| − | |align = "center"| 6894064, 6797719, 6458811, 5393763, 5457117, 5478847, 5811120, 5972383, 6906086, RE38968, RE39049, RE39050
| + | |
| − | |-
| + | |
| − | |align = "center"|20
| + | |
| − | |align = "center"|Detrol
| + | |
| − | |align = "center"|11/5/2020
| + | |
| − | |align = "center"|<nowiki>></nowiki>2014
| + | |
| − | |align = "center"| 5382600, 6630162, 6770295, 6911217
| + | |
| − | |-
| + | |
| − | |align = "center"|21
| + | |
| − | |align = "center"|Ditropan
| + | |
| − | |align = "center"|22/11/2015
| + | |
| − | |align = "center"|<nowiki>></nowiki>2014
| + | |
| − | |align = "center"|5674895, 5840754, 1 5912268, 6262115, 6919092
| + | |
| − | |-
| + | |
| − | |align = "center"|22 | + | |
| − | |align = "center"|Cialis | + | |
| − | |align = "center"|26/4/2020
| + | |
| − | |align = "center"|<nowiki>></nowiki>2014 | + | |
| − | |align = "center"|7182958, 5859006, 6140329, 6821975, 6943166
| + | |
| − | |-
| + | |
| − | |align = "center"|23
| + | |
| − | |align = "center"|Levitra
| + | |
| − | |align = "center"|31/10/2018
| + | |
| − | |align = "center"|<nowiki>></nowiki>2014
| + | |
| − | |align = "center"|6362178, 7696206
| + | |
| − | |-
| + | |
| − | |align = "center"|24
| + | |
| − | |align = "center"|Viagra
| + | |
| − | |align = "center"|22/10/2019
| + | |
| − | |align = "center"|<nowiki>></nowiki>2014
| + | |
| − | |align = "center"|6469012, 5250534
| + | |
| | |- | | |- |
| | |} | | |} |
| − | <br>
| |
| − | <br>
| |
| − | ==US Market Survey - Physicians Preferences and Insights==
| |
| − | ===Objectives of Survey===
| |
| − | * To find Physician preferences for OTC drugs in comparison to prescription drugs
| |
| − | * To find insights on OTC drugs market and usage
| |
| − | * To understand the effectiveness of different sources of marketing
| |
| − | ===Research Methodology===
| |
| − | The research instrument used was a questionnaire survey administered to collect empirical data. The physician responses were kept confidential to encourage openness and disclosure. The respondents rated the questions on Yes or No, ticked the relevant choices from the options available and ranked the options on a numerical scale. All the responses were coded using a binary system logic and analysis was done to derive insights there after.
| |
| − | ===Sample Size and Screening Criteria===
| |
| − | <br>
| |
| − | {|border="2" align = "center" cellspacing="0" cellpadding="4" width="35%"
| |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Gender'''
| |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Sample Size'''
| |
| − | |-
| |
| − | |align = "center"|Male
| |
| − | |align = "center"|18
| |
| − | |-
| |
| − | |align = "center"|Female
| |
| − | |align = "center"|2
| |
| − | |-
| |
| − | |align = "center" bgcolor = "#C5D9F1"|'''Total Physicians'''
| |
| − | |align = "center" bgcolor = "#C5D9F1"|'''20'''
| |
| − | |-
| |
| − | |}
| |
| − | <br>
| |
| − | {|border="2" align = "center" cellspacing="0" cellpadding="4" width="60%"
| |
| − | |align = "center" bgcolor = "#8DB4E3" colspan = "2"|'''Screening Criteria'''
| |
| − | |-
| |
| − | |align = "center"|Primary Medical Specialty
| |
| − | |align = "center"|General Physician/Family Practice
| |
| − | |-
| |
| − | |align = "center"|No. of years in practice
| |
| − | |align = "center"|10
| |
| − | |-
| |
| − | |align = "center"|No. of patients consulting per month
| |
| − | |align = "center"|150
| |
| − | |-
| |
| − | |}
| |
| − | <br>
| |
| | | | |
| − | ===Survey Results===
| + | [Source : Euromonitor International] |
| − | *All the respondents would recommend an OTC drug for fever
| + | |
| − | *About 95% of the doctors feel that the usage of OTC drugs has increased in the past five years
| + | |
| − | *About 85% of them would do so for other minor ailments like cold/cough and acidity
| + | |
| − | [[Image:USFirst.png|center|thumb|800px]] | + | |
| − | <br>
| + | |
| − | *Low cost, effective and easy availability are selected as the major factors that resulted in increased usage of OTC drugs
| + | |
| − | *All the respondents feel it is important to prescribe a drug that minimizes patients' out-of-pocket costs, while choosing between equally effective and safe medications
| + | |
| − | <br>
| + | |
| − | [[Image:US2.png|center|thumb|800px]]
| + | |
| − | *Advertisement is the major source of information on OTC drugs for most of the respondents
| + | |
| − | *Only 30% of respondents replied in positive when asked about permitting marketing executives to market OTC products through them
| + | |
| − | <br>
| + | |
| − | [[Image:US3.png|center|thumb|800px]]
| + | |
| − | *About 63% of the respondents reported the growth rate of OTC usage during 2005-2010 between 25% - 50% and equal percentage of respondents stated that patients are trusting OTC drugs more than they used to do before as the prime reason for the growth
| + | |
| − | {|align = "center"
| + | |
| − | |[[Image:US4.png|left|thumb|800px]][[Image:US5.png|right|thumb|800px]]
| + | |
| − | |-
| + | |
| − | |}
| + | |
| − | <br>
| + | |
| − | *Less than 50% of the patients are not prescribed OTC due to their medical history
| + | |
| − | [[Image:non pres due to medical history.jpg|center|thumb|800px]]
| + | |
| − | <br>
| + | |
| − | *The case of patients coming to the doctor due to side effects from OTC drugs happens once a month
| + | |
| − | *About 45% of the respondents feel that non-prescription of drugs is not a serious problem
| + | |
| − | {| align="center"
| + | |
| − | |[[Image:Seriousness of non-prescription1.jpg|left|thumb|488×299px]][[Image:side effects1.jpg|right|thumb|488×304px]]
| + | |
| − | |-
| + | |
| − | |}
| + | |
| | | | |
| − | <br>
| + | ===Categorization of Products=== |
| − | *85% of the doctors feel that the information on the label of OTC drugs is sufficient for the patients, in order to take OTC medicines
| + | |
| − | *50% of the doctors feel that more OTC drugs should be included under Medicare/insurance coverage
| + | |
| − | <br>
| + | |
| − | <br>
| + | |
| − | '''The effective ranking of the ailments in the order of safety of available OTC drugs is as follows:'''
| + | |
| − | [[Image:effective ranking.jpg|center|thumb|800px]]
| + | |
| − | [[Media:Working of Effective Ranking & Scoring.xls| To see the calculation of the effective scores and effective ranking, click on this link]]
| + | |
| | | | |
| − | ==Indian Market Survey - Physicians Preferences and Insights==
| + | * Here, we had tried to categorize the the different products under following 3 categories. |
| − | ===Objectives of the Survey===
| + | |
| | | | |
| − | The reason for carrying out this research is to determine the scope of potential switch of prescription drugs to over the counter drugs in the Indian pharma industry. The study is particularly focused at the scope of making statins(a drug for reducing cholesterol level) available over-the-counter. The study aims to understand how successful a switch would be, and if it is safe for such drugs to be made available without prescription. The study also aims at determining the marketing pattern of over the counter drugs, and how it can be improved in future.
| + | # Devices |
| | + | # Kits |
| | + | # Moisturizers/Lotion/cream |
| | | | |
| − | The objectives for the survey are :
| |
| | | | |
| − | * To understand the effectiveness of OTC drug marketing campaigns in India
| + | {|border="2" cellspacing="0" cellpadding="4" width="80%" |
| − | * To understand the source of information for the OTC drugs among doctors
| + | |bgcolor = "#FDE9D9"|Brand |
| − | * To explore the potential of switch from Rx to OTC drugs
| + | |bgcolor = "#FDE9D9"|Category |
| − | * To gauge the current prescription pattern of statins in India
| + | |
| − | * To identify if statins can be switched to OTC
| + | |
| | | | |
| − | ===Research Methodology===
| + | |- |
| − | The research instrument used was a questionnaire survey administered to collect empirical data. The physician responses were kept confidential to encourage openness and disclosure. The respondents rated the questions on Yes or No, ticked the relevant choices from the options available and ranked the options on a numerical scale. All the responses were coded using a binary system logic and analysis was done to derive insights there after.
| + | |Mentholatum Acnes(Oxy) |
| | + | |Moistureriser/Lotion/Cream |
| | | | |
| | + | |- |
| | + | |Clearasil Acne |
| | + | |Kits and Moistureriser/Lotion/Cream |
| | | | |
| | + | |- |
| | + | |Pair Acne |
| | + | |Moistureriser/Lotion/Cream |
| | | | |
| − | ===Sample Size and Screening Criteria===
| |
| − | '''Screening Criteria'''
| |
| − | {|border="2" align = "center" cellspacing="0" cellpadding="4" width="60%"
| |
| − | |align = "center" bgcolor = "#8DB4E3" colspan = "2"|'''Screening Criteria'''
| |
| | |- | | |- |
| − | |align = "center"|Medical Specialty | + | |Bif Night |
| − | |align = "center"|General Physician, Consulting Physician, Gynaecologist, Dermatologist, Cardiologist, Pediatrician
| + | |Moistureriser/Lotion/Cream |
| | + | |
| | |- | | |- |
| − | |align = "center"|No. of years in practice | + | |Pimplit |
| − | |align = "center"|10
| + | |Moistureriser/Lotion/Cream |
| | + | |
| | |- | | |- |
| − | |align = "center"|No. of patients consulting per month | + | |Menturm Acne Lotion |
| − | |align = "center"|150
| + | |Moistureriser/Lotion/Cream |
| | + | |
| | |- | | |- |
| − | |} | + | |Skinlife |
| | + | |Moistureriser/Lotion/Cream |
| | | | |
| − | <br>
| + | |- |
| | + | |Pair Acne Cream W |
| | + | |Moistureriser/Lotion/Cream |
| | | | |
| − | '''Sample Size'''
| |
| − | {|border="2" align = "center" cellspacing="0" cellpadding="4" width="25%" align = "center"
| |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Gender'''
| |
| − | |align = "center" bgcolor = "#8DB4E3"|'''Sample Size'''
| |
| | |- | | |- |
| − | |align = "center"|Male | + | |Acnepell |
| − | |align = "center"|44
| + | |Moistureriser/Lotion/Cream |
| | + | |
| | |- | | |- |
| − | |align = "center"|Female | + | |Eskamel |
| − | |align = "center"|16
| + | |Moistureriser/Lotion/Cream |
| | + | |
| | |- | | |- |
| − | |align = "center" bgcolor = "#C5D9F1"|'''Total Physicians''' | + | |Annsalbe |
| − | |align = "center" bgcolor = "#C5D9F1"|'''60'''
| + | |Moistureriser/Lotion/Cream |
| | + | |
| | |- | | |- |
| | |} | | |} |
| − | <br>
| |
| | | | |
| | + | ===Sales Forecast=== |
| | | | |
| − | {|border="2" cellspacing="0" cellpadding="4" width="25%" align = "center" | + | * The following image shows the forecasted sales figure of the acne treatment market for Japan. |
| − | |align = "center" bgcolor = "#95B3D7" colspan = "2"|'''Medical Specialty-wise Breakup''' | + | |
| | + | [[Image:Acne Treatment- Forecast.jpg|700px|thumb|center|Source : Euromonitor International]] |
| | + | |
| | + | |
| | + | |
| | + | ==<span style="color:#C41E3A">Like this report?</span>== |
| | + | <p align="center"> '''This is only a sample report with brief analysis''' <br> |
| | + | '''Dolcera can provide a comprehensive report customized to your needs'''</p> |
| | + | {|border="2" cellspacing="0" cellpadding="4" align="center" " |
| | + | |style="background:lightgrey" align = "center" colspan = "3"|'''[mailto:info@dolcera.com <span style="color:#0047AB">Buy the customized report from Dolcera</span>]''' |
| | |- | | |- |
| − | |General Physician | + | | align = "center"| [http://www.dolcera.com/website_prod/services/ip-patent-analytics-services Patent Analytics Services] |
| − | |align = "center"|17 | + | |align = "center"| [http://www.dolcera.com/website_prod/services/business-research-services Market Research Services] |
| | + | |align = "center"| [http://www.dolcera.com/website_prod/tools/patent-dashboard Purchase Patent Dashboard] |
| | |- | | |- |
| − | |Consulting Physician | + | |align = "center"| [http://www.dolcera.com/website_prod/services/ip-patent-analytics-services/patent-search/patent-landscapes Patent Landscape Services] |
| − | |align = "center"|9 | + | |align = "center"| [http://www.dolcera.com/website_prod/research-processes Dolcera Processes] |
| | + | |align = "center"| [http://www.dolcera.com/website_prod/industries Industry Focus] |
| | |- | | |- |
| − | |Gynecologist
| + | |align = "center"| [http://www.dolcera.com/website_prod/services/ip-patent-analytics-services/patent-search/patent-landscapes Patent Search Services] |
| − | |align = "center"|9 | + | |align = "center"| [http://www.dolcera.com/website_prod/services/ip-patent-analytics-services/alerts-and-updates Patent Alerting Services] |
| − | |-
| + | |align = "center"| [http://www.dolcera.com/website_prod/tools Dolcera Tools] |
| − | |Dermatologist
| + | |
| − | |align = "center"|4
| + | |
| − | |-
| + | |
| − | |Cardiologist
| + | |
| − | |align = "center"|12
| + | |
| − | |-
| + | |
| − | |Pediatrician
| + | |
| − | |align = "center"|6
| + | |
| − | |-
| + | |
| − | |align = "center" bgcolor = "#95B3D7" colspan = "2"|'''Geographywise Breakup'''
| + | |
| − | |-
| + | |
| − | |Mumbai
| + | |
| − | |align = "center"|15 | + | |
| − | |-
| + | |
| − | |Hyderabad
| + | |
| − | |align = "center"|15
| + | |
| − | |-
| + | |
| − | |Delhi NCR
| + | |
| − | |align = "center"|14
| + | |
| − | |-
| + | |
| − | |Bangalore
| + | |
| − | |align = "center"|16 | + | |
| | |- | | |- |
| | |} | | |} |
| | <br> | | <br> |
| | + | ==Contact Dolcera== |
| | | | |
| − | ===Survey Results===
| + | {| style="border:1px solid #AAA; background:#E9E9E9" align="center" |
| − | * 97% of the Indian doctors feel that the usage of OTC drugs has increased in the past 5 years
| + | |
| − | * Indian doctors feel that the usage of OTC drugs has increased by more than 50% in the past 5 years
| + | |
| − | * Ease in the availability of OTC drugs, Advertisements and Saving time are the major reasons for increase in the usage of OTC drugs
| + | |
| − | {|align = "center" width="100%" | + | |
| − | |[[Image:Opinion on increased usage - India1.jpg|left|thumb|520px]][[Image:Reasons for increase in OTC Usage - India.jpg|right|thumb|520px]]
| + | |
| | |- | | |- |
| − | |}
| + | ! style="background:lightgrey" | Samir Raiyani |
| − | <br>
| + | |
| − | * Headache, Cold/Cough and acidity are the most common ailments for which physicians prefer giving an OTC drug
| + | |
| − | [[Image:Physician preferences for minor ailments - india.jpg|center|thumb|603px]]
| + | |
| − | <br>
| + | |
| − | * More than 80% of the patients are not prescribed OTC drugs beacuse of their prior medical history
| + | |
| − | [[Image:non pres due to medical history - india.jpg|center|thumb|619px]]
| + | |
| − | <br>
| + | |
| − | * Only 13% of the doctors feel that the information on the labels of OTC drugs is sufficient
| + | |
| − | * 98% of the doctors feel that drug-drug interactions might be a potential danger for OTC drugs
| + | |
| − | * 48% of the patients come once a month to doctor due to side effects caused by OTC drugs
| + | |
| − | [[Image:Patients coming due to side effects - India.jpg|center|thumb|554px]]
| + | |
| − | <br>
| + | |
| − | * 95% of the doctors feel that the problem of taking OTCs in India is serious
| + | |
| − | [[Image:Seriousness of taking otc - india.jpg|center|thumb|547px]]
| + | |
| − | <br>
| + | |
| − | | + | |
| − | '''The effective ranking of the ailments in the order of safety of available OTC drugs is as follows:'''
| + | |
| − | [[Image:effective ranking - india.jpg|center|thumb|800px]]
| + | |
| − | [[Media:Working of Effective Ranking & Scoring - India.xls| To see the calculation of the effective scores and effective ranking, click on this link]]
| + | |
| − | <br>
| + | |
| − | | + | |
| − | * 72% of the doctors see an opportunity for Rx-to-OTC switch in Indian pharma market
| + | |
| − | * Drugs relating to Vitamins, Antacids, Cuts, wounds and burns & Cold/Cough have a better potential to do well as an OTC drug
| + | |
| − | [[Image:category with better potential for switch - india.jpg|center|thumb|570px]]
| + | |
| − | <br>
| + | |
| − | | + | |
| − | '''Effectiveness of marketing campaigns'''
| + | |
| − | * Advertisements are a major source of information for Indians on OTC drugs
| + | |
| − | [[Image:Source of info - india.jpg|center|thumb|514px]]
| + | |
| − | <br>
| + | |
| − | | + | |
| − | * Only 23% of the doctors permit marketing executives to market OTC products through them
| + | |
| − | * Quality is the most important factor affecting the choice of a drug
| + | |
| − | [[Image:factor affecting choice of a drug - india.jpg|center|thumb|484px]]
| + | |
| − | <br>
| + | |
| − | | + | |
| − | '''Results on Statins'''
| + | |
| − | * More than 50% of the patients with high cholesteral level fall in the age group of 40-60 years
| + | |
| − | * 73% of the patients with high cholesteral level are prescribed Statin by doctors
| + | |
| − | {|align = "center" width = "90%"
| + | |
| − | |[[Image:age grp increased cholesterol - india1.jpg|left|thumb|485px]][[Image:drug prescribed for cholesterol - india1.jpg|right|thumb|485px]]
| + | |
| | |- | | |- |
| | + | | '''Email''': [mailto:info@dolcera.com info@dolcera.com] |
| | + | |- |
| | + | | '''Phone''': +1-650-269-7952 |
| | |} | | |} |
| − | <br>
| |
| − | * Atrovastatin and Rosuvastatin are prescribed more than 90% of the times (whenever statins are prescribed) by doctors to patients with high cholesterol level
| |
| − | [[Image:prescribed statin - india.jpg|center|thumb|526px]]
| |
| − | <br>
| |
| − | * Sale and effectiveness of the drug, Manufacturer of the drug and Patient type are the major factors for choosing any type/brand of statin
| |
| − | [[Image:factors for choosing statin - india.jpg|center|thumb|565px]]
| |
| − | <br>
| |
| − | * Only 25% of the doctors feel that statins safe to be made available as OTC drugs for low- to-moderate risk group
| |
A global understanding of markets, products, and distribution channels related to the acne product market. Our overall focus is on the OTC market, not prescription.
Acne vulgaris (commonly called acne) is a common skin condition, affecting 85-100% of people at some time during their lives. It is caused by changes in the pilosebaceous units, skin structures consisting of a hair follicle and its associated sebaceous gland. It is characterized by noninflammatory follicular papules or comedones and by inflammatory papules, pustules, and nodules in its more severe forms. Acne vulgaris affects the areas of skin with the densest population of sebaceous follicles; these areas include the face, the upper part of the chest, and the back. Severe acne is inflammatory, but acne can also manifest in noninflammatory forms. Acne lesions are commonly referred to as pimples, blemishes, spots, zits, or acne. Source
There's been a gap in products marketed to adult women--little or nothing was available to address their acne needs, and as a result, sales have lagged. "No one was looking to target the 30- to 40-year-olds," said Lee Feldman, vice president of sales for Advanced Research Labs. Source
Acne is a common occurrence at puberty. With the increased production of sex hormones, the sebaceous glands become hyperactive.
Androgen and progesterone are responsible for the hyperplasia of the oil glands.
The usual premenstrual flare-up is explained by some observers as occurring during the period when the normal androgen-estrogen balance in the blood is altered in favor of androgen. Hence hormonal imbalance is held responsible for causing acne.
Psychogenic stresses particularly the habit of picking pimples, makes them worse.
Besides seborrhoeic diathesis and hormonal imbalance, other factors which aggravate acne are:
Medical treatment is suggested only in cases of chronic or severe acne and should be taken from the dermatologist.
Benzoyl Peroxide preparations available as gels and lotions have become very popular in the treatment of acne. Cream "Ultra Clearasil" is a very potent Benzoyl preparation. One of the main action points of Benzoyl Peroxide is to kill bacteria. It releases oxygen which helps in killing aerobic bacteria.
The sulphur drug preparations, available as creams, gels and lotions, are extremely effective in treating acne. Like Benzoyl Peroxide, sulphur is committed to killing bacteria. Sulphur is a disinfectant and used in skin treatments for eczema, etc.
Tetracycline usually has no side-effects and hence is the safest drug. However, in some uncommon cases, it causes bilousness, indigestion, vaginal thrush or rash.
Secondary data sources are only used to conduct the research.
As per BCC Research the value of the Global Acne Medications Market for year 2012 was estimated to be $3.5 billion and it is expected to reach $4.5 billion by year 2018 at a CAGR of 2.8%.