Difference between pages "Wind Energy" and "Backup"
(→Contact Dolcera) |
(→Methodology) |
||
| Line 1: | Line 1: | ||
| − | + | [[Wiki section]] | |
| − | [[ | + | |
| − | + | ==Process to Identify Possible Acquisition Targets== | |
| − | = | + | |
| − | + | ||
| − | + | ||
| − | + | Before identifying the possible acquisition targets, first companies needs to identify the objective of the merger and acquisition. | |
| − | + | ||
| − | + | ||
| − | + | ===Advantage of Mergers and Acquisitions=== | |
| − | + | The most common motives and advantages of mergers and acquisitions are:- | |
| − | + | *Accelerating a company's growth, particularly when its internal growth is constrained due to paucity of resources. Internal growth requires that a company should develop its operating facilities- manufacturing, research, marketing, etc. But, lack or inadequacy of resources and time needed for internal development may constrain a company's pace of growth. Hence, a company can acquire production facilities as well as other resources from outside through mergers and acquisitions. Specially, for entering in new products/markets, the company may lack technical skills and may require special marketing skills and a wide distribution network to access different segments of markets. The company can acquire existing company or companies with requisite infrastructure and skills and grow quickly. | |
| − | + | ||
| − | + | *Enhancing profitability because a combination of two or more companies may result in more than average profitability due to cost reduction and efficient utilization of resources. This may happen because of:- | |
| + | **Economies of scale:- arise when increase in the volume of production leads to a reduction in the cost of production per unit. This is because, with merger, fixed costs are distributed over a large volume of production causing the unit cost of production to decline. Economies of scale may also arise from other indivisibilities such as production facilities, management functions and management resources and systems. This is because a given function, facility or resource is utilized for a large scale of operations by the combined firm. | ||
| + | **Operating economies:- arise because, a combination of two or more firms may result in cost reduction due to operating economies. In other words, a combined firm may avoid or reduce over-lapping functions and consolidate its management functions such as manufacturing, marketing, R&D and thus reduce operating costs. For example, a combined firm may eliminate duplicate channels of distribution, or crate a centralized training center, or introduce an integrated planning and control system. | ||
| + | **Synergy:- implies a situation where the combined firm is more valuable than the sum of the individual combining firms. It refers to benefits other than those related to economies of scale. Operating economies are one form of synergy benefits. But apart from operating economies, synergy may also arise from enhanced managerial capabilities, creativity, innovativeness, R&D and market coverage capacity due to the complementarity of resources and skills and a widened horizon of opportunities. | ||
| − | + | *Diversifying the risks of the company, particularly when it acquires those businesses whose income streams are not correlated. Diversification implies growth through the combination of firms in unrelated businesses. It results in reduction of total risks through substantial reduction of cyclicality of operations. The combination of management and other systems strengthen the capacity of the combined firm to withstand the severity of the unforeseen economic factors which could otherwise endanger the survival of the individual companies. | |
| − | + | ||
| − | * | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | *A merger may result in financial synergy and benefits for the firm in many ways:- | |
| + | **By eliminating financial constraints | ||
| + | **By enhancing debt capacity. This is because a merger of two companies can bring stability of cash flows which in turn reduces the risk of insolvency and enhances the capacity of the new entity to service a larger amount of debt | ||
| + | **By lowering the financial costs. This is because due to financial stability, the merged firm is able to borrow at a lower rate of interest. | ||
| − | + | *Limiting the severity of competition by increasing the company's market power. A merger can increase the market share of the merged firm. This improves the profitability of the firm due to economies of scale. The bargaining power of the firm vis-à-vis labour, suppliers and buyers is also enhanced. The merged firm can exploit technological breakthroughs against obsolescence and price wars. | |
| − | * | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | '''Note:''' In this research report, researcher considered two following objective of merger and acquisition: | |
| + | #To Enter in emerging markets | ||
| + | #To improve product portfolio | ||
| − | + | ===Methodology=== | |
| − | + | * '''Step 1''': First, list all the players present in Ureteral Stent Market were identified.(The list of companies was retrieved from the FDA site from Registration & Listing database) | |
| − | + | ** Number of companies that were identified in this step: 20 | |
| − | * | + | |
| − | + | * '''Step 2''': The second step involved identifying and eliminating companies that are large, and established players or the subsidiaries of the big players in the industry. | |
| + | **Number of companies eliminated in this step: 14 | ||
| + | **Number of (small) companies of interest left: 6 | ||
| − | * | + | * '''Step 3''': Once the large, and established players were eliminated, companies were compared based on various parameters and rated on the scale of 5 to identify the best target. Please check the following table and dashboard: |
| − | + | **Number of potential target companies: 6 | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | * | + | |
| − | + | ||
| − | + | <br/> | |
| − | == | + | {|border="2" cellspacing="0" cellpadding="4" width="100%" align="center" |
| − | + | |align = "center" bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Company'''</font> | |
| − | + | |align = "center" bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Headquartered'''</font> | |
| − | + | |align = "center" bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Stent Details'''</font> | |
| − | + | |align = "center" bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Company Type'''</font> | |
| − | + | |align = "center" bgcolor = "#4F81BD"|<font color="#FFFFFF">'''2010 Revenue (Mn)'''</font> | |
| − | + | |align = "center" bgcolor = "#4F81BD"|<font color="#FFFFFF">'''No of Employees'''</font> | |
| − | + | |align = "center" bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Geographical Revenue Share'''</font> | |
| − | + | |align = "center" bgcolor = "#4F81BD"|<font color="#FFFFFF">'''R&D investment (Mn)'''</font> | |
| − | + | |align = "center" bgcolor = "#4F81BD"|<font color="#FFFFFF">'''No. of Patent'''</font> | |
| + | |align = "center" bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Technology Focus'''</font> | ||
| + | |- | ||
| + | |bgcolor = "#DBE5F1"|Applied Medical Resources Corp. | ||
| + | |bgcolor = "#DBE5F1"|USA | ||
| + | |bgcolor = "#DBE5F1"|Mesh ureteral stent<br>C-flex ureteral stent<br>Silicone ureteral stent<br>Tethered ureteral stent<br>C-flex ureteral stent<br>Ureteral stent<br>Silhouette pediatric stent | ||
| + | |bgcolor = "#DBE5F1"|Manufacturer | ||
| + | |align = "center" bgcolor = "#DBE5F1"|$282 | ||
| + | |align = "center" bgcolor = "#DBE5F1"|1900 | ||
| + | |bgcolor = "#DBE5F1"|USA: 62%<br>Asia: 32%<br>Rest of World: 6% | ||
| + | |bgcolor = "#DBE5F1"|$38.3 (13%) | ||
| + | |align = "center" bgcolor = "#DBE5F1"|212 | ||
| + | |bgcolor = "#DBE5F1"|High | ||
| + | |- | ||
| + | |Bioteque Corp. | ||
| + | |Taiwan | ||
| + | |Bioteque Double Pigta | ||
| + | |Contract Manufacturer; Contract Sterilizer; Manufacturer | ||
| + | |align = "center"|$27.85 | ||
| + | |align = "center"|350 | ||
| + | |Taiwan: 100% | ||
| + | |$1.4 (5%) | ||
| + | |align = "center"|16 | ||
| + | |Medium | ||
| + | |- | ||
| + | |bgcolor = "#DBE5F1"|Hobbs Medical, Inc. | ||
| + | |bgcolor = "#DBE5F1"|USA | ||
| + | |bgcolor = "#DBE5F1"|HM Ureteral Double Pigtail Stent | ||
| + | |bgcolor = "#DBE5F1"|Manufacturer | ||
| + | |align = "center" bgcolor = "#DBE5F1"|$2.30 | ||
| + | |align = "center" bgcolor = "#DBE5F1"|22 | ||
| + | |bgcolor = "#DBE5F1"|USA: 97.2%<br>Japan: 2.3%<br>Others: 0.5% | ||
| + | |bgcolor = "#DBE5F1"|$0.06 (2.87%) | ||
| + | |align = "center" bgcolor = "#DBE5F1"|1 | ||
| + | |bgcolor = "#DBE5F1"|Low | ||
| + | |- | ||
| + | |Lake Region Medical Limited | ||
| + | |Ireland | ||
| + | |M-Wires | ||
| + | |Contract Manufacturer | ||
| + | |align = "center"|$84.86 | ||
| + | |align = "center"|611 | ||
| + | |Europe: 68%<br>USA: 21%<br>Others: 11% | ||
| + | |$8.48 (3.85%) | ||
| + | |align = "center"|8 | ||
| + | |Medium | ||
| + | |- | ||
| + | |bgcolor = "#DBE5F1"|Martech Medical Products | ||
| + | |bgcolor = "#DBE5F1"|USA | ||
| + | |bgcolor = "#DBE5F1"|Ureteral Stent | ||
| + | |bgcolor = "#DBE5F1"|Contract Manufacturer | ||
| + | |align = "center" bgcolor = "#DBE5F1"|$23.00 | ||
| + | |align = "center" bgcolor = "#DBE5F1"|180 | ||
| + | |bgcolor = "#DBE5F1"|USA: 78.3%<br>Europe: 12.7%<br>Others: 9% | ||
| + | |bgcolor = "#DBE5F1"|$1.1 (4.79%) | ||
| + | |align = "center" bgcolor = "#DBE5F1"|0 | ||
| + | |bgcolor = "#DBE5F1"|Low | ||
| + | |- | ||
| + | |Allium Medical | ||
| + | |Israel | ||
| + | |URS - Ureteral Stent<br>TPS - Triangular Prostatic Stent<br>BUS - Bulbar Urethral Stent<br>RPS - Round Posterior Urethral Stent<br>BIS - Biliary Stent | ||
| + | |Manufaturer | ||
| + | |align = "center"|$2.04 | ||
| + | |align = "center"|20 | ||
| + | |Domestic: 88.6%<br>International: 11.4% | ||
| + | |$0.082 (4%) | ||
| + | |align = "center"|2 | ||
| + | |Low | ||
| + | |- | ||
| + | |}<br clear="all"> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | {|align="center" | |
| − | + | |<gflash>1000 750 http://dolcera.com/upload/files/ranking1.swf</gflash> | |
| − | + | |- | |
| − | + | |} | |
| − | + | <br/> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | |||
| − | |||
| − | + | * '''Step 4''': Finally, after an in-depth analysis of the potential target companies’ on various parameters, following companies identified to be best possible target companies. | |
| − | + | **To improve Product Portfolio : '''Applied Medical Resources Corp.''' | |
| − | + | **To enter in Emerging Markets : '''Bioteque Corp.''' | |
| − | + | ||
| − | + | ||
| − | + | ===Company Profile=== | |
| + | ====Applied Medical Resources Corp.==== | ||
| − | + | {|border="2" cellspacing="0" cellpadding="4" width="60%" align="center" | |
| − | + | |bgcolor = "#376091" colspan = "2"|<font color="#FFFFFF">'''APPLIED MEDICAL RESOURCES CORP.'''</font> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | {|border="2" cellspacing="0" cellpadding="4" width=" | + | |
| − | |bgcolor = "# | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#C5D9F1"|'''Revenues''' |
| − | | | + | |bgcolor = "#C5D9F1"|2010: $280 million |
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | + | |'''Net profit (2010)''' | |
| − | | | + | |$20.89 million |
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#C5D9F1"|'''R&D Investment''' |
| − | | | + | |bgcolor = "#C5D9F1"|2010: $ 38.3 million (13% of revenues) |
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | + | |'''Number of employees''' | |
| − | | | + | |1900 |
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#C5D9F1"|'''Year Established''' |
| − | | | + | |bgcolor = "#C5D9F1"|1987 |
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | + | |'''Headquarters''' | |
| − | | | + | |USA |
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#C5D9F1"|'''Key People''' |
| − | | | + | |bgcolor = "#C5D9F1"|CEO: Said S. Hilal |
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | + | |'''Products & Technology''' | |
| − | | | + | |Specialty Areas:Cardiac/Vascular, Colorectal, GYN, Urology <br>New Products:Epix (Laparoscopic Intrumentation), GelPOINT (Advanced Access Platform), Kii Fios (First EntrySystem) |
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#C5D9F1"|'''Products in Ureteral Stent''' |
| − | | | + | |bgcolor = "#C5D9F1"|C-flex ureteral stent, Silicone ureteral stent, Tethered ureteral stent, C-flex ureteral stent, Ureteral stent<br>Silhouette pediatric stent |
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | + | |'''Geographical revenue breakdown (2010)''' | |
| − | | | + | |USA: $182.70 million (65%)<br>Others: $99.47 million (35%) |
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | | | + | |bgcolor = "#C5D9F1"|'''Company Overview''' |
| + | |bgcolor = "#C5D9F1"|Applied Medical Resources Corporation is a new generation medical device company founded in 1987 and headquartered in Southern California. It is involved in developing, manufacturing and marketing of innovative products for Minimally Invasive Surgery, Cardiac, Vascular, Urological, Colorectal, Bariatric, Obstetric, Gynecologic and General Surgery. The product portfolio covers 25 technologies and more than 700 products. The company has spread its business globally across 75 countries including Africa, Middle East,Americas, Caribbean, Asia, Australia and Europe through its network of international distributors. | ||
|- | |- | ||
| − | |} | + | |} |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ====Bioteque Corp.==== | |
| − | + | ||
| − | + | {|border="2" cellspacing="0" cellpadding="4" width="60%" align="center" | |
| − | {|border="2" cellspacing="0" cellpadding="4" width=" | + | |bgcolor = "#376091" colspan = "2"|<font color="#FFFFFF">'''Bioteque Corp.'''</font> |
| − | | | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#C5D9F1"|'''Revenues''' |
| − | |bgcolor = "# | + | |bgcolor = "#C5D9F1"|2010: $27.85 million |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | | | + | |'''Net profit (2010)''' |
| − | | | + | |$20.89 million |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#C5D9F1"|'''R&D Investment''' |
| − | |bgcolor = "# | + | |bgcolor = "#C5D9F1"|2010: $38.3 million (13% of revenues) |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | | | + | |'''Number of employees''' |
| − | | | + | |350 |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#C5D9F1"|'''Year Established''' |
| − | |bgcolor = "# | + | |bgcolor = "#C5D9F1"|1991 |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | | | + | |'''Headquarters''' |
| − | | | + | |Taiwan |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#C5D9F1"|'''Key People''' |
| − | |bgcolor = "# | + | |bgcolor = "#C5D9F1"| |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | | | + | |'''Products & Technology''' |
| − | | | + | |<br> NEPHROLOGY, UROLOGY, RADIOLOGY, CARDIOLOGY, RESPIRATORY CARE<br> CRITICAL CARE, IV ADMINISTRATION THERAPY, MOLDING PARTS |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | | | + | |bgcolor = "#C5D9F1"|'''Products in Ureteral Stent''' |
| + | |bgcolor = "#C5D9F1"|C-flex ureteral stent, Silicone ureteral stent, Tethered ureteral stent, C-flex ureteral stent, Ureteral stent<br>Silhouette pediatric stent | ||
|- | |- | ||
| − | | | + | |'''Geographical revenue breakdown (2010)''' |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|NA | |NA | ||
| − | |- | + | |- |
| − | + | |bgcolor = "#C5D9F1"|'''Company Overview''' | |
| − | + | |bgcolor = "#C5D9F1"|Bioteque Corporation manufactures and sells medical devices in Taiwan. It offers medical disposables for use in hemodialysis access, endovascular treatment, and other fields. It offers blood tubing lines, AVF needles, transducer protectors, and on line HDF with check valves or without check valves; IV infusion bags, precision IV infusion sets, drainage bags, insufflation tubing sets/filters, and various surgical drainage tubes; a range of medical components, which comprise blood tubing line components, percutaneous drainage components, infusion bag components, AVF needle components, precision IV infusion set components, and IV infusion bag components; a range of thermoplastic polyurethane catheters, including pigtail drainage catheter sets, double pigtail ureteral stent sets, biliary drainage catheters, percutaneous nephrostomy kits, and dialysis catheters; and other medical disposable products, such as closed suction catheters and artificial nose. The company also provides endovascular products, which consist of percutaneous transluminal coronary angioplasty, percutaneous transluminal angioplasty, angiography catheters, guiding catheters, sheath introducers, MRI/CT/angiography syringes, micro catheters, and hydrophilic coated guidewires. Bioteque Corporation was founded in 1991 and is based in Taipei, Taiwan. | |
| − | + | ||
| − | |bgcolor = "# | + | |
| − | | | + | |
| − | + | ||
| − | + | ||
| − | | | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
|} | |} | ||
| − | = | + | ==M&A Due Diligence Process== |
| − | == | + | == Phase 1: Landscape overview == |
| − | + | === Ureteral Stent: Concept === | |
| − | === | + | An antimicrobial ureteral stent, which inhibits encrustation and bacterial colonization while maintaining patient comfort. |
| + | |||
| + | * Ureteral stent: resists migration, resists fragmentation, is kink resistant and radiopaque. | ||
| + | * Bacterial colonization: antimicrobial activity for up to two weeks. | ||
| + | * Patient Comfort: stent has a low coefficient of fiiction (value) for ease of insertion and will soften on implant at body temperature to maintain patient comfort. | ||
| + | |||
| + | === Background === | ||
| + | Ureteral stents are used in urological surgery to maintain patency of the ureter to allow urine drainage from the renal pelvis to the bladder. These devices can be placed by a number of different endourological techniques. They are typically inserted through a cystoscope and may also be inserted intraoperatively. Indwelling ureteral stents help to reduce complications and morbidity subsequent to urological and surgical procedures. Frequently, ureteral stents are used to facilitate drainage in | ||
| + | conjunction with Extracorporeal Shock Wave Lithotripsy (ESWL) and after endoscopic procedures. They are also used to internally support anastomoses and prevent urine leakage after surgery. Ureteral stenting may almost eliminate the urological complications of renal transplantation. | ||
| + | |||
| + | The advent of ESWL and the more recent barrage of endourological techniques have increased the indications for ureteral stents (Candela and Bellman 1997). Indications for use include: | ||
| + | * Treatment of ureteral or kidney stones | ||
| + | * Ureteral trauma or stricture | ||
| + | * Genitourinary reconstructive surgery | ||
| + | * Hydronephrosis during pregnancy | ||
| + | * Obstruction due to malignancy | ||
| + | * Retroperitoneal fibrosis | ||
| + | |||
| + | The need for ureteral stents range from a few days to several months. For patients with serious urological problems, ureteral stent maintenance may become a life-long necessity. Unfortunately, there are many problems associated with using ureteral stents. | ||
| + | |||
| + | === Ureteric stenting difficulties === | ||
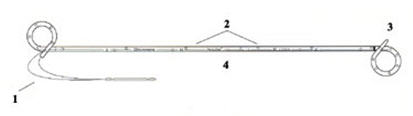
| + | [[Image:Ureteral stent.jpg|thumb|right|350px|Double-J and Pigtail ureteral stents]] | ||
| + | {|border="2" cellspacing="2" cellpadding="4" width="50%" | ||
| + | |align = "center" bgcolor = "#00CCFF"|<font color="#993366">'''Common'''</font> | ||
| + | |align = "center" bgcolor = "#00CCFF"|<font color="#993366">'''Rare'''</font> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
| + | | | ||
| + | * Trigonal irritation | ||
| + | * Haematuria | ||
| + | * Fever | ||
| + | * Infection | ||
| + | * Tissue inflammation | ||
| + | * Encrustation | ||
| + | * Biofilm formation | ||
| + | | | ||
| + | * Obstruction | ||
| + | * Kinking | ||
| + | * Ureteric rupture | ||
| + | * Ureteric perforation | ||
| + | * Stent misplacement | ||
| + | * Stent migration | ||
| + | * Stent misfit | ||
| + | * Stent forgotten | ||
| + | * Tissue hyperplasia | ||
|} | |} | ||
| − | + | Today, elastomeric materials, such as silicones, polyurethanes and hydrogel-coated polyolefins are used, with no clear winner, which can withstand the urinary environment. | |
| + | * Although silicone has better long-term stability than other stent materials, its extreme flexibility makes it difficult to pass over guidewires and through narrow or tortuous ureters. | ||
| + | * Polyethylene is stiffer and easier to use for patients with strictures; however, it has been known to become brittle with time leading to breakage and is no longer commercially available. * Polyurethane has properties that fall in between polyethylene and silicone; however, stent fracture also has been an issue with polyurethanes. | ||
| − | + | Attempts have been made to develop polymers with a combination of the best of all properties. The key players are C-Flex (Concept Polymer Technologies), Silitek and Percuflex (Boston Scientific). | |
| − | + | * C-Flex is proprietary silicone oil and mineral oil interpenetrated into a styrenelolefin block copolymer with the hope of reduced encrustation. | |
| − | + | * Silitek (Medical Engineering Corporation) is another silicone-based copolymer. | |
| − | + | * Percuflex is a proprietary olefinic block copolymer. | |
| − | + | ||
| − | + | Metallic stents have been used recently to treat extrinsic ureteric obstructions. The effect of synthetic polymers on the urothelium of the urinary tract seems to be dependent on the bulk chemical composition of the polymer, the chemical composition of its surface, coatings on the device | |
| − | + | surface, smoothness of the surface and coefficient of friction. | |
| − | + | ||
| − | + | Typically, most ureteral stents are made of relatively smooth catheters. [http://www.ncbi.nlm.nih.gov/pubmed/10772512 Koleski et al., (2000)] tested a longitudinally grooved ureteral stent made by Circon in the pig ureter. The results indicated that the grooved stent led to better drainage than a conventional stent. Their opinion is that the ureter wall has a better chance of collapsing over a | |
| − | + | smooth surface than a grooved surface, especially when debris is present. Stoller (2000) had the same experience with the SpiraStent(Urosurge Corp.). This helical stent was superior at passing stones than a conventional smooth stent. | |
| − | + | ||
| − | + | There are a variety of ureteral stent configurations with different anchoring systems. Most stents today have a double [http://linkinghub.elsevier.com/retrieve/pii/S014067360002674X pigtail anchoring system]. (Tolley, 2000), Dunn et al, (2000) conducted a randomized, single-blind study comparing a Tail stent (proximal pigtail with a shaft which tapers to a lumenless straight tail) to a double pigtail stent. The Tail stent was found to be better tolerated than the double-pigtail concerning | |
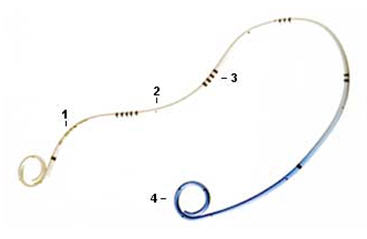
| − | + | lower urinary tract irritative symptoms. A double-J ureteral stent and a flexible ureteropyeloscope are shown in the first diagram. The other two diagrams show a pigtail ureteral stent in place; the end of the pigtail is facing away fiom the ureteral opening in the second of these two diagrams. | |
| − | + | ||
| − | + | Early adverse effects of ureteral stenting include lower abdominal pain, dysuria, fever, urinary frequency, nocturia and hematuria. Patient discomfort and microscopic hematuria happen often. Major late complications include stent migration, stent fragmentation or more serious hydronephrosis with flank pain and infections. | |
| − | + | ||
| − | + | Late complications occurred in one third of the patients in a prospective study using both silicone and polyurethane double pigtail stents (110 stents) in 90 patients. Stent removal was necessary in these patients. Others also have found this percentage of late complications. Device-related urinary tract infection and encrustation can lead to significant morbidity and even death and are the primary factors limiting long-term use of indwelling devices in the urinary tract. Microbial biofilm and encrustation may lead to stone formation. This is typically not a problem when stents are used | |
| − | + | for short-term indications. Problems of biofilm formation, encrustation and stent fracture occur in patients with long-term indwelling stents. | |
| − | + | ||
| − | + | Typically, manufacturers advise periodic stent evaluation. Cook polyurethane stent removal is recommend at 6 months and 12 months for silicone (Cook product literature). However, stents that are intended for long-term use are usually changed at regular intervals, as frequently as every 3 months. | |
| − | + | ||
| − | + | Forgotten stents are a problem. Monga et al., 1995 found that 68% of stents forgotten more than 6 months were calcified and 10% were fragmented. Multiple urologic procedures were necessary to remove the stones. Long-term effects of these forgotten stents may lead to voiding dysfunction and renal insufficiency. Schlick, et al., 1998 are developing a biodegradable stent that will preclude the need for stent removal. | |
| − | + | ||
| − | + | ==== Encrustation ==== | |
| − | + | The urinary system presents a challenge because of its chemically unstable environment. Long-term biocompatibility and biodurability of devices have been problems due to the supersaturation of uromucoids and crystalloids at the interface between urine and the device. Encrustation of ureteral stents is a well-known problem, which can be treated easily if recognized early. However, severe encrustation leads to renal failure and is difficult to manage (Mohan-Pillai et al., 1999). All biomaterials currently used become encrusted to some extent when exposed to urine. | |
| − | + | ||
| − | + | The encrusted deposits can harbor bacterial biofilms. In addition, they can render the biomaterial brittle which causes fracture in-situ, a serious problem especially associated with the use of polyethylene and polyurethane ureteral stents (although silicone stents have also been | |
| − | + | reported to fracture). Stent fragments can migrate to the bladder or renal pelvis with serious repercussions. | |
| − | + | ||
| − | + | Surface science techniques were used to study three stent types after use in patients. The stent type, duration of insertion and age or sex of the patient did not correlate significantly with the amount of encrustation (Wollin et al., 1998). However, it has been suggested that factors which affect the amount of encrustation include the composition or the urine, the type of invading and colonizing bacteria and the structure and surface properties of the biomaterial used (Gorman 1995). A low surface energy surface seems to resist encrustation compared with a high surface energy surface (Denstedt et al., 1998). | |
| − | + | ||
| − | |- | + | Many different types of stone can form in the urinary tract. Calcium oxalate, calcium phosphate, uric acid and cystine stones are metabolic stones because they form as a result of metabolic dysfunction. They usually are excreted from the urinary tract. Struvite (magnesium ammonium phosphate) and hydroxyapatite (calcium phosphate) are associated with infection (infection stones). These account for 1520% of urinary calculi. ESWL is used to break up the larger infection stones because they don't pass; recurrence of the problem occurs with incomplete removal. Infection stones can manifest as poorly mineralized matrix stones, highly mineralized staghorn calculi or as bladder stones which often form in the presence of ureteral stents. Urea-splitting bacteria colonize the surface and cause alkalinization of the urine, which lowers the solubility of struvite and hydroxyapatite, and they deposit on the surface. Bacterial biofilm associated with encrustation is a common clinical occurrence. (Gorman and Tunney, 1997). It has been suggested that prevention of bacterial colonization would prevent encrustation because of their ultimate responsibility for its formation (Bibby et al., 1995). |
| − | + | ||
| − | + | An in vitro model was developed that produces encrustation similar to those seen in vivo (Tunney et al., 1996a). An experiment was conducted to compare the encrustation potential of various ureteral stent materials. The long-term struvite and hydroxyapatite encrustation of silicone, polyurethane, hydrogel-coated polyurethane, Silitek and Percuflex were compared. All of the materials developed encrustation, however, it was found by image analysis that the rates of encrustation varied on the different materials. Silicone had less encrustation (69% at 10 weeks) compared to the other materials (1 00%) at the same time point (Tunney et al., 1996b). Continuous flow models have also been developed which are more representative of conditions in the upper urinary tract. They are discussed by Gorman and Tunney, (1 997). Efforts to reduce encrustation using new materials, smoother | |
| − | | | + | surfaces and hydrogel coatings have been attempted. |
| − | + | ||
| − | + | A hydrogel-coated C-flex stent (Hydroplus, Boston Scientific) was shown to have less epithelial cell damage and encrustation than other biomaterials and was recommended by the investigators for long-term use (Cormio, 1995). In addition, a poly(ethy1ene oxide)/polyurethane composite hydrogel (Aquavenem, J & J) resisted intraluminal blockage in a urine flow model compared with silicone and polyurethane (Gorman et al., 1997a). Another advantage with Aquavene is that it is rigid in the dry state, which facilitates insertion past obstructions in the ureter and becomes soft on hydration providing comfort (Gorman and Tunney, 1997). Gorman et al. (1997b) concluded that the chance of stent fracture would be reduced if the ureteral stent side holes were eliminated. Urinary tract infection is another common major problem with the usage of ureteral stents. Initially, a conditioning film is deposited on the ureteral stent surface. The film is made up of proteins, electrolyte materials and other unidentified materials that obscure the surface properties of the stent material. Electrostatic interactions, the ionic strength and pH of the urine and differences in fluid surface tensions affect bacterial adhesion to the conditioning film. Subsequently, a microbial biofilm forms over time. The biofilm is composed of bacterial cells embedded in a hydrated, predominantly anionic mixture of bacterial exopolysaccharides and trapped host extracellular macromolecules. | |
| − | + | ||
| − | |bgcolor = "# | + | ====Obstruction==== |
| − | + | Obstruction of urine flow and urinary tract sepsis can result in continued growth of the biofilm. Colonization of devices implanted in the urinary tract can lead to dysfunction, tissue intolerance, pain, subclinical or overt infection and even urosepsis. Device related infections are difficult to | |
| − | + | treat and device removal is usually necessary. The biofilm has been found to impede the diffusion of antibiotics; in addition, the bacteria in the biofilm have a decreased metabolic rate , which also protects them against the effects of antibiotics (Wollin et al., 1998). Riedl, et al. (1 999) found 100% ureteral stent colonization rates in permanent and 69.3% in temporary stents. Antibiotic prophylaxis did not prevent bacterial colonization and it was recommended that it not be used. | |
| − | | | + | On the other hand, Tieszer, et al. (1 998) believe that fluoroquinolones can prevent infection. They also have found that some stents have denser encrustation than others, however, the stent material did not change the elements of the "conditioning film" adsorbed or alter its receptivity to |
| − | | | + | bacterial biofilms. |
| − | | | + | |
| − | | | + | ====Infection==== |
| − | | | + | The predictive value of urine cultures in the assessment of stent colonization was examined in 65 patients with indwelling ureteral stents. It was found that a sterile urine culture did not rule out the stent itself being colonized (Lifshitz, et al., 1999). Patients with sterile urine culture |
| − | |- | + | may benefit from prophylactic antibiotics; however, the authors contended that the antibiotics must work against gram-negative uropathogens and gram-positive bacteria including enterococci. |
| − | | | + | It is obvious that there is controversy in the literature whether prophylactic systemic antibiotics are useful with ureteral stent implant. However, antibiotics do not seem to prevent stent colonization. Denstedt et al. (1998) have found that ciprofloxacin, with a 3 day burst every 2 |
| − | + | weeks, actually is adsorbed onto the stent which makes longer term treatment possible with reduced risk of bacterial resistance. There has been research targeted at coating or impregnating urinary catheters with antimicrobials and products are on the market, however, there are no antimicrobial ureteral stents approved by the FDA. | |
| − | | | + | |
| − | | | + | === The market need === |
| + | It is clear that there is a need for a new material that will be able to resist encrustation and infection in the urinary tract. According to Merrill Lynch, ureteral stents represent an $80 MM | ||
| + | US market. Boston Scientific is in the lead with ~50% of the market followed by Maxxim (Circon), Cook and Bard is a smaller player. There are a number of other small contenders. | ||
| + | |||
| + | The use of ureteral stents is increasing; the indications for ureteral stenting have broadened from temporary or permanent relief or ureteric obstruction to include temporary urinary diversion following surgical procedures such as endopyelotomy and ureteroscopy and facilitation of stone clearance after ESWL (Tolley, 2000). | ||
| + | |||
| + | The use of ureteral stents for patients having ESWL for renal calculi is however controversial and seems to be related to the size of the stones and invasiveness of the procedure. According to survey results reported by Hollowell, et al. (2000), there is a significant difference in opinion concerning the use of stents with ESWL. | ||
| + | |||
| + | The number of ureteral stents used in patients with stones 2 cm or less treated with ESWL is significant in spite of the lack scientific evidence in support of this practice. Of 1,029 urologists returning surveys, for patients with renal pelvic stones 10, 15 or 20 rnm treated with ESWL, routine stent placement was preferred by 25.3%, 57.1 % and 87.1 %, respectively. Urologists recommend using ureteroscopy rather than ESWL for distal ureteral calculi 5-1 0 mm. | ||
| + | |||
| + | === Intellectual property === | ||
| + | ==== Search strategy ==== | ||
| + | * Databases searched: US-G, US-A, EP-A, EP-B, WO, JP, DE, GB, FR | ||
| + | * Search scope: Title, Abstract or Claims | ||
| + | * Years: 1981-July 2008 | ||
| + | * Search query: (ureter* OR urether* OR ureth* OR uretr*) AND (stent*) AND (*microb* OR *bacter*) | ||
| + | * Results: '''177 patents (82 unique patent families)''' | ||
| + | |||
| + | ==== Sample patents ==== | ||
| + | {| class="wikitable" style="font-size:90%" border="1" cellpadding="5" cellspacing="0" | ||
| + | |- style="background:lightgrey" | ||
| + | !align = "center" bgcolor = "#00CCFF"|Patent | ||
| + | !bgcolor = "#00CCFF"|Assignee | ||
| + | !bgcolor = "#00CCFF"|Title | ||
| + | !bgcolor = "#00CCFF"|Abstract | ||
| + | |- | ||
| + | ![http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2Fsrchnum.htm&r=1&f=G&l=50&s1=6468649.PN.&OS=PN/6468649&RS=PN/6468649 US6468649 B1] | ||
| + | | SCIMED LIFE SYSTEMS INC | ||
| + | | Antimicrobial adhesion surface | ||
| + | | The present invention provides an implantable medical device having a substrate with a hydrophilic coating composition to limit in vivo colonization of bacteria and fungi. The hydrophilic coating composition includes a hydrophilic polymer with a molecular weight in the range from about 100, 000 to about 15 million selected from copolymers acrylic acid, methacrylic acid, isocrotonic acid and combinations thereof. | ||
| + | |- | ||
| + | ![http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2Fsrchnum.htm&r=1&f=G&l=50&s1=5554147.PN.&OS=PN/5554147&RS=PN/5554147 US5554147 A] | ||
| + | | CApHCO, Inc. | ||
| + | | Compositions and devices for controlled release of active ingredients | ||
| + | | A method for the controlled release of a biologically active agent wherein the agent is released from a hydrophobic, pH-sensitive polymer matrix is disclosed and claimed. The polymer matrix swells when the environment reaches pH 8.5, releasing the active agent. A polymer of hydrophobic and weakly acidic comonomers is disclosed for use in the controlled release system. Further disclosed is a specific embodiment in which the controlled release system may be used. The pH-sensitive polymer is coated onto a latex catheter used in ureteral catheterization. A common problem with catheterized patients is the infection of the urinary tract with urease-producing bacteria. In addition to the irritation caused by the presence of the bacteria, urease produced by these bacteria degrade urea in the urine, forming carbon dioxide and ammonia. The ammonia causes an increase in the pH of the urine. Minerals in the urine begin to precipitate at this high pH, forming encrustations which complicate the functioning of the catheter. A ureteral catheter coated with a pH-sensitive polymer having an antibiotic or urease inhibitor trapped within its matrix will release the active agent when exposed to the high pH urine as the polymer gel swells. Such release can be made slow enough so that the drug remains at significant levels for a clinically useful period of time. | ||
| + | |- | ||
| + | ![http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220030153983%22.PGNR.&OS=DN/20030153983&RS=DN/20030153983 US20030153983 A1] | ||
| + | | SCIMED LIFE SYSTEMS INC | ||
| + | |Implantable or insertable medical device resistant to microbial growth and biofilm formation | ||
| + | | Disclosed are implantable or insertable medical devices that provide resistance to microbial growth on and in the environment of the device and resistance to microbial adhesion and biofilm formation on the device. In particular, the invention discloses implantable or insertable medical devices that comprise at least one biocompatible matrix polymer region, an antimicrobial agent for providing resistance to microbial growth and a microbial adhesion/biofilm synthesis inhibitor for inhibiting the attachment of microbes and the synthesis and accumulation of biofilm on the surface of the medical device. Also disclosed are methods of manufacturing such devices under conditions that substantially prevent preferential partitioning of any of said bioactive agents to a surface of the biocompatible matrix polymer and substantially prevent chemical modification of said bioactive agents | ||
|- | |- | ||
|} | |} | ||
| − | === | + | ==Urinary Problems in men and women== |
| + | * Both men and women have an increased risk for urinary incontinence as they get older, with men's rates rising steadily and women's rates peaking during menopause. | ||
| + | * The prevalence of incontinence in men of all ages is certainly lower than that for women. | ||
| + | * Women over 70, however, are twice as likely to have urinary incontinence as men of the same age. | ||
| + | |||
| + | [http://www.ehow.com/facts_5664964_urinary-prevalence-men-vs_-women.html Source: Urinary prevalence men Vs women] | ||
| + | |||
| + | |||
| + | ==Market Analysis== | ||
| + | * We determined market data to have an idea about the market potential for ureteral stents. | ||
| + | * We have done this modeling for female population in US as women has the higher prevalence rate for urinary incontinence than men in all age groups. | ||
| + | * Prevalence increased with age, from 28% for 30- to 39-year-old women to 55% for 80- to 90-year-old women. | ||
| + | * 18% of respondents reported severe UI. | ||
| + | * The prevalence of severe UI also increased notably with age, from 8% for 30- to 39-year-old women to 33% for 80- to 90-year-old women. | ||
| + | * Among all, 9% reported slight UI, 15% reported moderate UI, 18% reported severe UI, and 58% reported no UI. | ||
| + | <BR> | ||
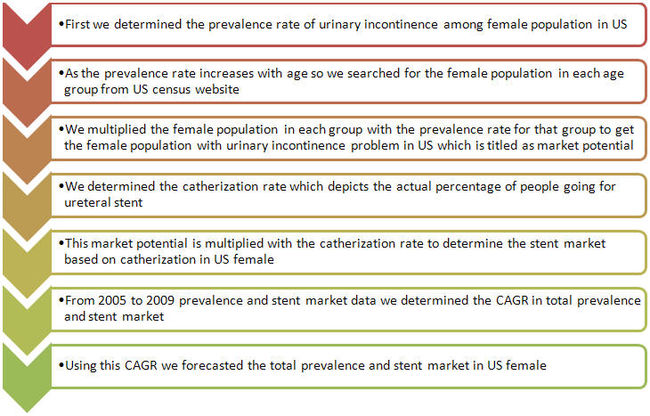
| + | ===Methodology=== | ||
| + | [[Image:methodology-ureteral stents.jpg|center|650px]] | ||
| + | |||
| + | ===Prevalence rate in US (women)=== | ||
{|border="2" cellspacing="0" cellpadding="4" width="100%" | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| − | |align = "center" bgcolor = "#4F81BD" | + | |align = "center" bgcolor = "#4F81BD" colspan = "2"|<font color="#FFFFFF">'''Prevalence of Urinary Incontinence in US (women)'''</font> |
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#D3DFEE"|Age (in yrs) |
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#D3DFEE"|Population with Urinary incontinence (in %) |
| − | + | ||
|- | |- | ||
| − | |align = "center | + | |align = "center"|30-39 |
| − | + | |align = "center"|28% | |
| − | + | ||
| − | | | + | |
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#D3DFEE"|40-49 |
| − | | | + | |align = "center" bgcolor = "#D3DFEE"|41% |
| − | + | ||
| − | | | + | |
|- | |- | ||
| − | |align = "center | + | |align = "center"|50-59 |
| − | + | |align = "center"|48% | |
| − | + | ||
| − | | | + | |
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#D3DFEE"|60-69 |
| − | | | + | |align = "center" bgcolor = "#D3DFEE"|51% |
| − | + | ||
| − | | | + | |
|- | |- | ||
| − | |align = "center | + | |align = "center"|70-79 |
| − | + | |align = "center"|55% | |
| − | + | ||
| − | | | + | |
|- | |- | ||
| − | + | |align = "center" bgcolor = "#D3DFEE"|80-90 | |
| − | + | |align = "center" bgcolor = "#D3DFEE"|54% | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | |align = "center" bgcolor = "# | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | |align = "center" bgcolor = "# | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
|} | |} | ||
| − | + | [http://archinte.ama-assn.org/cgi/content/full/165/5/537 Source: Archives of internal medicine] | |
| − | + | ||
| − | + | ===Urinary incontinence severity among different age groups in US women=== | |
| − | + | ||
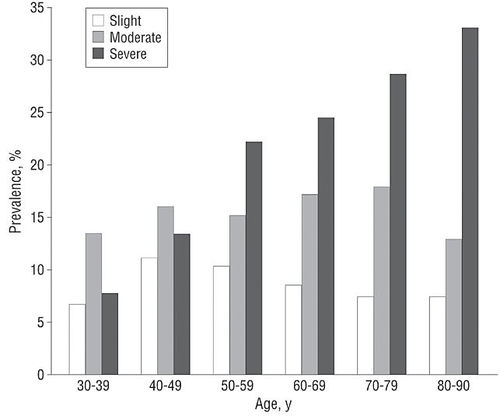
| − | + | [[Image:urinary incontinence severity.jpg|center|500px|thumb|[http://archinte.ama-assn.org/cgi/content/full/165/5/537 Source: Archives of internal medicine]]] | |
| − | + | ||
| + | ===Market potential for ureteral stent in US (women)=== | ||
| − | |||
| − | |||
| − | |||
| − | |||
{|border="2" cellspacing="0" cellpadding="4" width="100%" | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| − | + | |align = "center" bgcolor = "#4F81BD" colspan = "6"|<font color="#FFFFFF">'''Market potential for ureteral stents in US women, 2009'''</font> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | |align = "center" bgcolor = "#4F81BD" colspan = " | + | |
|- | |- | ||
| − | | | + | |bgcolor = "#D3DFEE"|1. Age groups<br> |
| − | + | |bgcolor = "#D3DFEE"|2. Female population<br>(from US census data) | |
| − | + | |bgcolor = "#D3DFEE"|3. Prevalence rate in female (%) | |
| − | + | |align = "center" bgcolor = "#D3DFEE"|4. Market potential<br>(total prevalence)<br>(2<nowiki>*</nowiki>3) | |
| − | |bgcolor = "# | + | |bgcolor = "#D3DFEE"|5. Catherization rate (%)<br> |
| − | | | + | |align = "center" bgcolor = "#D3DFEE"|6. Stent market based on catherization rate<br>(4<nowiki>*</nowiki>5) |
| − | + | |- | |
| − | + | |30-39 | |
| − | | | + | |20128402 |
| − | + | |28% | |
| − | + | |5635953 | |
| − | + | |0.043% | |
| − | + | |2423 | |
| − | + | |- | |
| − | + | |bgcolor = "#D3DFEE"|40-49 | |
| − | + | |bgcolor = "#D3DFEE"|22074384 | |
| − | + | |bgcolor = "#D3DFEE"|41% | |
| − | + | |bgcolor = "#D3DFEE"|9050497 | |
| − | + | |bgcolor = "#D3DFEE"|0.123% | |
| − | + | |bgcolor = "#D3DFEE"|11132 | |
| − | |bgcolor = "# | + | |- |
| − | |align = "center" bgcolor = "# | + | |50-59 |
| − | + | |20929761 | |
| − | + | |48% | |
| − | + | |10046285 | |
| − | + | |0.124% | |
| − | + | |12457 | |
| − | + | |- | |
| − | + | |bgcolor = "#D3DFEE"|60-69 | |
| − | | | + | |bgcolor = "#D3DFEE"|14605565 |
| − | | | + | |bgcolor = "#D3DFEE"|51% |
| − | | | + | |bgcolor = "#D3DFEE"|7448838 |
| − | | | + | |bgcolor = "#D3DFEE"|0.160% |
| − | | | + | |bgcolor = "#D3DFEE"|11918 |
| − | | | + | |- |
| − | | | + | |70-79 |
| − | |bgcolor = "# | + | |9046207 |
| − | | | + | |55% |
| − | |bgcolor = "# | + | |4975414 |
| − | |bgcolor = "# | + | |0.172% |
| − | |bgcolor = "# | + | |8558 |
| − | |bgcolor = "# | + | |- |
| − | |- | + | |bgcolor = "#D3DFEE"|≥ 80 |
| − | | | + | |bgcolor = "#D3DFEE"|7216598 |
| − | + | |bgcolor = "#D3DFEE"|54% | |
| − | | | + | |bgcolor = "#D3DFEE"|3896963 |
| − | | | + | |bgcolor = "#D3DFEE"|0.044% |
| − | | | + | |bgcolor = "#D3DFEE"|1715 |
| − | | | + | |- |
| − | | | + | |'''Total''' |
| − | |- | + | | |
| − | | | + | | |
| − | + | |'''41053950''' | |
| − | | | + | | |
| − | |bgcolor = "# | + | |'''48203''' |
| − | |bgcolor = "# | + | |
| − | |bgcolor = "# | + | |
| − | |bgcolor = "# | + | |
| − | |- | + | |
| − | | | + | |
| − | + | ||
| − | | | + | |
| − | | | + | |
| − | | | + | |
| − | | | + | |
| − | | | + | |
| − | |- | + | |
| − | | | + | |
| − | |bgcolor = "# | + | |
| − | | | + | |
| − | |bgcolor = "# | + | |
| − | |bgcolor = "# | + | |
| − | + | ||
| − | |bgcolor = "# | + | |
| − | |- | + | |
| − | | | + | |
| − | | | + | |
| − | | | + | |
| − | | | + | |
| − | | | + | |
| − | | | + | |
| − | + | ||
|- | |- | ||
|} | |} | ||
| − | |||
| − | === | + | |
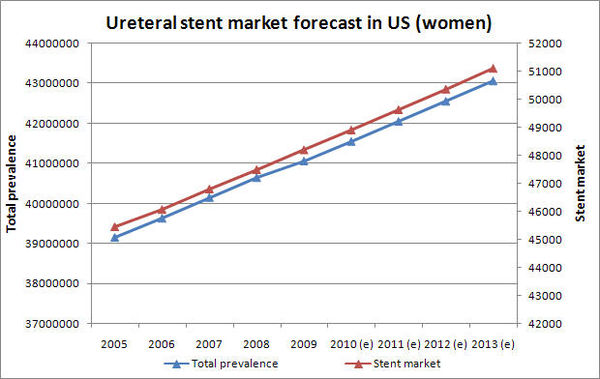
| − | + | * Prevalence rate in US women is growing at a CAGR of 1.19% | |
| − | + | * Ureteral stent market is growing at a CAGR of 1.47% | |
| − | | | + | <Br> |
| − | + | ||
| − | + | ===Ureteral stent market forecast in US (women)=== | |
| − | + | [[Image:stent market forecast1.jpg|center|600px]] | |
| − | + | ||
| − | + | [[Media:Detailed calculation workbook.xls|'''Detailed model workbook''']] | |
| − | + | ||
| − | | | + | ===Ureteral stent companies=== |
| − | | | + | Various companies offering ureteral stents are: |
| − | | | + | |
| − | + | * [http://www.bostonscientific.com/Device.bsci/,,/method/DevHome/navRelId/1000.1003/seo.serve Boston Scientific Corporation] | |
| − | | | + | * [http://www.cookmedical.com/uro/familyListingAction.do?family=Ureteral+Stents Cook Group] |
| − | | | + | * [http://www.appliedmed.com/products/product_card.aspx?prodGroupID=6&catID=37&Name=Ureteral+stents Applied Medicals] |
| − | | | + | * [http://www.redi-tech.com/products/?get=pricing&loc=main&content=0&type=pricing&items=0&item=13 Redi-Tech Medical Products] |
| − | + | ||
| − | | | + | ====Ureteral stents of various companies==== |
| − | + | =====Boston Scientific===== | |
| − | | | + | * Contour VL Variable Length Percuflex Stents<BR> |
| − | | | + | Inward spiral design of Nautilus Coil minimizes tissue contact for enhanced comfort. Dual variable length coil geometry permits balanced stent positioning to minimize the risk of migration. HydroPlus Coating provides unequalled surface lubricity which reduces friction to minimize risk of buckling during introduction and placement and to reduce risk of trauma and encrustation. |
| − | + | ||
| − | | | + | [[Image:Contour VL variable length percuflex stents.jpg|center|500px|thumb|[http://www.bostonscientific.com/Device.bsci/,,/method/DevHome/navRelId/1000.1003/seo.serve Source: www.bostonscientific.com]]] |
| − | + | ||
| − | + | * Percuflex Stents<Br> | |
| − | |align = "center"| | + | :* High coil strength pigtail shape prevent stent migration |
| − | + | :* Thin wall promotes drainage and patency | |
| − | |align = "center"| | + | :* Multiple, large side ports promote drainage |
| − | + | :* Attached suture for positioning and subsequent removal without the need for repeat cystoscopy | |
| − | + | [[Image:percuflex stents.jpg|center|500px|thumb|[http://www.bostonscientific.com/Device.bsci/,,/method/DevHome/navRelId/1000.1003/seo.serve Source: www.bostonscientific.com]]] | |
| − | |- | + | |
| − | |align = "center | + | * Polaris Ultra Ureteral Stent |
| − | | | + | :* Ultra Ureteral Stent provides the ease of placement benefits of a firm stent graduated into a soft bladder coil |
| − | |align = "center | + | :* Co-extrusion combines a firm durometer Percuflex Plus Material and a soft durometer Percuflex Material in the same stent |
| − | | | + | :* Nautilus bladder coil potentially reduce bladder irritation, and a relaxed renal coil facilitate ease of removal |
| − | | | + | |
| + | [[Image:Polaris Ultra Ureteral Stent.jpg|center|500px|thumb|[http://www.bostonscientific.com/Device.bsci/,,/method/DevHome/navRelId/1000.1003/seo.serve Source: www.bostonscientific.com]]] | ||
| + | |||
| + | =====Cook Group===== | ||
| + | * Bander Ureteral Diversion Stent Set | ||
| + | It is used for intraoperative placement to stent the ureter during ureteroileal conduit construction and continent urinary diversions. Set includes: 2 stents, 2 catheter retainers and wire guide. | ||
| + | |||
| + | [[Image:Bander Ureteral Diversion Stent Set.jpg|center|500px|thumb|[http://www.cookmedical.com/uro/familyListingAction.do?family=Ureteral+Stents Source: www.cookmedical.com]]] | ||
| + | |||
| + | * C-Flex Double Pigtail Ureteral Stent Set | ||
| + | It is used for temporary internal drainage from the ureteropelvic junction to the bladder. Set includes stent, wire guide, stent positioner and catheter. | ||
| + | |||
| + | [[Image:C-Flex Double Pigtail Ureteral Stent Set.jpg|center|500px|thumb|[http://www.cookmedical.com/uro/familyListingAction.do?family=Ureteral+Stents Source: www.cookmedical.com]]] | ||
| + | |||
| + | * Towers Peripheral Ureteral Stent Set | ||
| + | It is also used for temporary internal drainage from the ureteropelvic junction to the bladder. The stent configuration allows peripheral as well as luminal drainage. Set Includes: Stent, Wire Guide, Catheter, and Stent Positioner. | ||
| + | |||
| + | [[Image:Towers Peripheral Ureteral Stent Set.jpg|500px|center|thumb|[http://www.cookmedical.com/uro/familyListingAction.do?family=Ureteral+Stents Source: www.cookmedical.com]]] | ||
| + | |||
| + | =====Applied medicals===== | ||
| + | * 7-10 endopyelotomy stent | ||
| + | It is used by urologists for endopyelotomy and endoureterotomy. The dual diameter promotes optimal healing while minimizing the discomfort often associated with larger diameter stents. | ||
| + | |||
| + | [[Image:ureteral stent-applied.jpg|center|500px|thumb|[http://www.appliedmed.com/products/product_card.aspx?prodGroupID=6&catID=37&Name=Ureteral+stents Source: www.appliedmed.com]]] | ||
| + | |||
| + | =====Redi-Tech Medical Products===== | ||
| + | * Ureteral stents set | ||
| + | :* Attached suture for positioning and subsequent removal without the need for repeat cystoscopy | ||
| + | :* Multiple, large side ports promote drainage | ||
| + | :* Radiopaque stent markings aid in placement and sizing | ||
| + | |||
| + | [[Image:Ureteral stent-redi tech.jpg|center|400px|thumb|[http://www.redi-tech.com/products/?get=pricing&loc=main&content=0&type=pricing&items=0&item=13 Source: www.redi-tech.com]]] | ||
| + | |||
| + | ==<span style="color:#C41E3A">Like this report?</span>== | ||
| + | <p align="center"> '''This is only a sample report with brief analysis''' <br> | ||
| + | '''Dolcera can provide a comprehensive report customized to your needs'''</p> | ||
| + | {|border="2" cellspacing="0" cellpadding="4" align="center" " | ||
| + | |style="background:lightgrey" align = "center" colspan = "3"|'''[mailto:info@dolcera.com <span style="color:#0047AB">Buy the customized report from Dolcera</span>]''' | ||
| + | |- | ||
| + | | align = "center"| [http://www.dolcera.com/website_prod/services/ip-patent-analytics-services Patent Analytics Services] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/services/business-research-services Market Research Services] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/tools/patent-dashboard Purchase Patent Dashboard] | ||
| + | |- | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/services/ip-patent-analytics-services/patent-search/patent-landscapes Patent Landscape Services] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/research-processes Dolcera Processes] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/industries Industry Focus] | ||
| + | |- | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/services/ip-patent-analytics-services/patent-search/patent-landscapes Patent Search Services] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/services/ip-patent-analytics-services/alerts-and-updates Patent Alerting Services] | ||
| + | |align = "center"| [http://www.dolcera.com/website_prod/tools Dolcera Tools] | ||
|- | |- | ||
|} | |} | ||
| − | |||
<br> | <br> | ||
| − | + | === Clinical Trials === | |
| − | === | + | ====New trials ==== |
| − | + | {| {{table}} | |
| − | + | | align="center"|'''Title''' | |
| − | | | + | | align="center"|'''Conditions''' |
| − | |align = "center | + | | align="center"|'''Intervention''' |
| − | |align = "center | + | | align="center"|'''Sponsors and Collaborators''' |
| − | |align = "center | + | |- |
| − | |align = "center | + | | [http://clinicaltrials.gov/ct2/show/NCT00250406?term=ureteral+stent&rank=1 Assessment of Drug-Eluting Ureteral Stent on Bacterial Adherence and Biofilm Formation]||Renal Calculi, Ureteral Obstruction||Device: Ureteral Stent||Lawson Health Research Institute, Boston Scientific Corporation |
| − | |- | + | |- |
| − | | | + | | [http://clinicaltrials.gov/ct2/show/NCT00270504?term=urethral+stent&rank=1 Memokath® 044TW Stent for Treatment of Urethral Stricture]||Urethral Stricture||Device: Memokath stenting||Engineers & Doctors Wallsten Medical Group |
| − | + | |- | |
| − | | | + | | [http://clinicaltrials.gov/ct2/show/NCT00581178?term=urologic+stent&rank=3 Study to Determine if There Are Specific Clinical Factors to Determine Stent Encrustation]||Kidney Stones||N\A||University of California, Irvine |
| − | | | + | |- |
| − | | | + | | [http://clinicaltrials.gov/ct2/show/NCT00288457?term=urologic+stent&rank=14 Ureteral Stent Length and Patient Symptoms]||Kidney Stones||Device: Ureteral Stent||Emory University |
| − | + | |- | |
| − | |- | + | | [http://clinicaltrials.gov/ct2/show/NCT00166361?term=urologic+stent&rank=1 Drainage of Malignant Extrinsic Ureteral Obstruction Using the Memokath Ureteral Stent]||Ureteral Obstruction||Device: Memokath 051 Ureteral Stent||Mayo Clinic Engineers & Doctors Wallsten Medical Group |
| − | | | + | |- |
| − | + | | [http://clinicaltrials.gov/ct2/show/NCT00739284?term=urologic+stent&rank=15 A Prospective Comparison Between Ureteral Stent and Nephrostomy Tube for an Urgent Drainage of Obstructed Kidney (JJVsPCN08)]||Kidney Disease||Device: nephrostomy tube and ureteral stent||Rabin Medical Center | |
| − | | | + | |
| − | | | + | |
| − | | | + | |
| − | | | + | |
| − | |- | + | |
| − | | | + | |
| − | + | ||
| − | | | + | |
| − | | | + | |
| − | | | + | |
| − | + | ||
| − | |- | + | |
| − | | | + | |
| − | + | ||
| − | | | + | |
| − | | | + | |
| − | | | + | |
| − | | | + | |
| − | |- | + | |
| − | | | + | |
| − | + | ||
| − | | | + | |
| − | | | + | |
| − | | | + | |
| − | + | ||
| − | |- | + | |
| − | | | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | | | + | |
| − | | | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | | | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
|} | |} | ||
| − | === | + | ==== Concluded trials ==== |
| + | {| {{table}} | ||
| + | | align="center" |'''Title''' | ||
| + | | align="center" |'''Abstract''' | ||
| + | | align="center" |'''Enrollment''' | ||
| + | | align="center" |'''Disorder''' | ||
| + | | align="center" |'''Conclusion''' | ||
| + | |- | ||
| + | | Long-term outcome of permanent urethral stents in the treatment of detrusor-sphincter dyssynergia ||To evaluate the long-term efficacy of a permanently implanted urethral stent in the treatment of spinally injured patients with detrusor-sphincter dyssynergia.||13||Detrusor-sphincter dyssynergia||Stenting is an effective alternative to sphincterotomy in the long-term, although secondary bladder neck obstruction is a frequent problem. | ||
| + | |- | ||
| + | | Nephrostomy Tube or 'JJ' Ureteric Stent in Ureteric Obstruction: Assessment of Patient Perspectives Using Quality-of-Life Survey and Utility Analysis||Upper urinary tract obstruction is often relieved by either a percutaneous nephrostomy tube (PCN) or a ureteric stent. Both can cause considerable morbidity and reduce patient's health-related quality of life (QoL). We have compared the QoL in these 2 groups.||34||Upper urinary tract obstruction||Patients with 'JJ' stents have significantly more irritative urinary symptoms and a high chance of local discomfort than patients with nephrostomy tubes (PCN). However, based on the EuroQol analysis, there is no significant difference in the gross impact on the health-related QoL or the utility between these groups indicating no patient preference for either modality of treatment. | ||
| + | |- | ||
| + | | Impact of stents on urological complications and health care expenditure in renal transplant recipients: results of a prospective, randomized clinical trial.||A randomized, prospective trial to compare the incidence of early urological complications and health care expenditures in renal transplant recipients with or without ureteral stenting.||201||Renal transplant recipient||Using a ureteral stent at renal transplantation significantly decreases the early urinary complications of urine leakage and obstruction. However, there is a significant increase in urinary tract infections, primarily beyond 30 days after transplantation. Stent removal within 4 weeks of insertion appears advisable. | ||
| + | |} | ||
| + | ====Adverse Events==== | ||
{|border="2" cellspacing="0" cellpadding="4" width="100%" | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#00CCFF"|'''S. No.''' |
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#00CCFF"|'''Brand Name''' |
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#00CCFF"|'''Adverse Event''' |
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#00CCFF"|'''Date FDA Received''' |
| − | |align = "center" bgcolor = "# | + | |- |
| − | + | |align = "center" bgcolor = "#00CCFF"|'''1''' | |
| − | |align = " | + | |align = "justify"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/Detail.cfm?MDRFOI__ID=660847 Cook Urologicals Cook Urological Stent]</u></font> |
| − | + | |align = "justify"|Stent broke into pieces while removing it from the patients body. | |
| − | + | |align = "center"|12/14/2005 | |
| − | + | |- | |
| − | + | |align = "center" bgcolor = "#00CCFF"|'''2''' | |
| − | + | |align = "justify"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/Detail.CFM?MDRFOI__ID=930422 Boston Scoientific Boston Scientific Ureteral stent System]</u></font> | |
| − | + | |align = "justify"|Fractured stent seen under Fluroscopy | |
| − | + | |align = "center"|10/17/2007 | |
| − | |align = " | + | |- |
| − | + | |align = "center" bgcolor = "#00CCFF"|'''3''' | |
| − | |align = "center"| | + | |align = "justify"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/Detail.CFM?MDRFOI__ID=755260 Boston Scoientific Boston Scientific Ureteral Stent System Kit 8 FR X 24 CM]</u></font> |
| − | |- | + | |align = "justify"|During insertion of ureteral stent, the stent broke into multiple parts which were retained in the patient. |
| − | |align = "center" bgcolor = "# | + | |align = "center"|10/14/2005 |
| − | | | + | |- |
| − | + | |align = "center" bgcolor = "#00CCFF"|'''4''' | |
| − | + | |align = "justify"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/Detail.CFM?MDRFOI__ID=564910 Boston Scientific Corp Boston Scientific 8 FR X 28 CM Ureteral Stent System Kit]</u></font> | |
| − | + | |align = "justify"|Breakage of the upper loop of the ureteral stent while trying to insert it. | |
| − | + | |align = "center"|1/5/2005 | |
| − | + | |- | |
| − | + | |align = "center" bgcolor = "#00CCFF"|'''5''' | |
| − | |align = " | + | |align = "justify"|<font color="#0000FF"><u>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/Detail.CFM?MDRFOI__ID=522129 Boston Scientific Bostoon Scientific Micro Vasive Contour VL Ureteral Stent]</u></font> |
| − | + | |align = "justify"|Broken stent observed during x-ray procedure. | |
| − | |align = "center"| | + | |align = "center"|12/12/2003 |
| − | |- | + | |
| − | |align = "center" bgcolor = "# | + | |
| − | | | + | |
| − | |align = " | + | |
| − | + | ||
| − | |align = "center | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | |- | + | |
| − | |align = "center" bgcolor = "# | + | |
| − | | | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | |align = " | + | |
| − | + | ||
| − | |align = "center"| | + | |
| − | |- | + | |
| − | |align = "center" bgcolor = "# | + | |
| − | | | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | |align = " | + | |
| − | + | ||
| − | |align = "center"| | + | |
|- | |- | ||
|} | |} | ||
| + | [[Media: non patent upload.xls|'''Review Articles''']]<br> | ||
| + | [[Media: non patent upload1.xls|'''Non Patent Analysis''']] | ||
| − | === | + | === Products === |
| − | + | {| {{table}} | |
| − | + | | align="center"|'''Boston Scientific Scimed, Inc.''' | |
| − | + | | align="center"|'''Cook Urological Incorporated''' | |
| − | {| | + | | align="center"|'''OptiMed Global Care''' |
| − | | | + | |- |
| − | | | + | | Polaris™ Ultra Ureteral Stent |
| − | | | + | | Firlit-Kluge Urethral Stent |
| − | | | + | | Opti-J Ureteral Stent System |
| − | | | + | |- |
| − | | | + | | Polaris™ Loop Ureteral Stent |
| − | | | + | | Koyle Diaper Stent |
| − | | | + | | Ureteral Stent Sets, ureterorenoscope |
| − | | | + | |- |
| − | | | + | | Stretch™ VL Variable Length Flexima® Stents |
| − | | | + | | Silicone Universal Drainage Stent |
| − | | | + | | Extra Strong Stent Sets (-Tumor) |
| − | | | + | |
| − | | | + | |
| − | + | ||
|- | |- | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| | | | ||
| + | | Tarkington Urethral Stent Set | ||
| + | | Steerable Ureteral Stent Sets | ||
| + | |- | ||
| | | | ||
| − | | | + | | Zaontz Urethral Stent |
| − | | | + | | Multilength |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| | | | ||
| + | | Pediatric Urethral C-Stent | ||
| | | | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | === Startup activity === | ||
| + | * [http://twincities.bizjournals.com/twincities/stories/2008/07/28/story8.html AbbeyMoor Medical Inc.], a med-tech firm that’s developed devices for treating urological disorders, has raised $2.7 million in bridge financing. | ||
| + | |||
| + | == Phase 2: Deeper Dive == | ||
| + | === Scenario === | ||
| + | Client wishes to acquire a ureteral stent company. | ||
| + | |||
| + | === Deal analysis for a target company === | ||
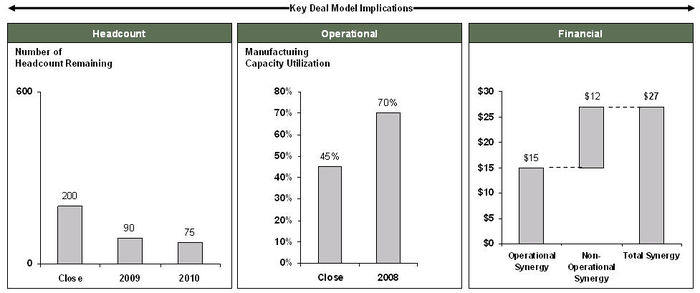
| + | [[Image:DealImplications.jpg|thumb|center|700px|Deal implications]] | ||
| + | |||
| + | === Design History File Review: Review components === | ||
| + | {| class="wikitable" style="font-size:90%" border="1" cellpadding="5" cellspacing="0" | ||
| + | |- style="background:lightgrey" | ||
| + | !align = "center" bgcolor = "#00CCFF" width="15%"|Review | ||
| + | !bgcolor = "#00CCFF" width="30%"|Verification | ||
| + | !bgcolor = "#00CCFF" width="40%"|Tasks | ||
| + | !bgcolor = "#00CCFF" width="15%"|Expertise | ||
| + | |- | ||
| + | !rowspan="2"|Design Input | ||
| + | | Design input documents for sufficiency | ||
| + | | | ||
| + | # Access electronic data room. | ||
| + | # Check what documents are provided. | ||
| + | # Compare document list with standard client document list. | ||
| + | # Check whether each specified document has appropriate content. | ||
| + | | rowspan="2"|Quality systems | ||
| + | |- | ||
| + | | Design input documents linked to the product performance specifications | ||
| | | | ||
| + | # Compare product specifications to design inputs | ||
| + | # Check whether appropriate verifications and validations are performed | ||
| + | # Establish if all specifications are linked to design inputs | ||
| + | |- | ||
| + | !rowspan="3"|Product Performance Specifications (PPS) | ||
| + | | Design inputs correlate adequately to the specifications; DV&V (design verification and validation) criteria are based on risk management documentation or if the criteria are based on sound statistical sampling plans | ||
| + | | | ||
| + | # Compare product specifications to design inputs | ||
| + | # Check whether appropriate verifications and validations are performed | ||
| + | # Establish if all specifications are linked to design inputs | ||
| + | | rowspan="3"|Quality systems, CAD | ||
| + | |- | ||
| + | | Appropriate design verification and validations (DV&V) are performed | ||
| + | | | ||
| + | # Show DV&V criteria are based on risk management requirements | ||
| + | |- | ||
| + | | Product performance specifications correspond to appropriate design output documents | ||
| + | | | ||
| + | # Correlate design drawings with the specifications | ||
| + | # Check whether maximum dimensions, sizes etc. (with tolerances) are within the specified range | ||
| + | |- | ||
| + | !rowspan="4"|Risk Management Documents | ||
| + | | Risk Analysis, Design Failure Modes and Effects Analysis (DFMEA), Process FMEA, other risk management documentation | ||
| + | | | ||
| + | # Check whether documentation is available | ||
| + | # Check whether it adheres to appropriate ISO 14971 standards | ||
| + | # Check whether it adheres to appropriate client standards | ||
| + | |rowspan="4"| Quality systems | ||
| + | |- | ||
| + | | DFMEA links appropriately to the PPS | ||
| + | | | ||
| + | # Verify whether DFMEA and product specifications are correlated | ||
| + | |- | ||
| + | | Appropriate DV&V reports and design output documents are referenced correctly as risk mitigation activities in the DFMEA | ||
| + | | | ||
| + | # Validate the process and correlate with design inputs | ||
| + | # Validate that sizes used are within range of risk mitigation criteria | ||
| + | |- | ||
| + | | PFMEA links appropriately to the process validation protocol acceptance criteria; In-process inspection procedures and/or manufacturing procedures are recorded as appropriate risk mitigation activities in the PFMEA | ||
| + | | | ||
| + | # Validate the process protocol | ||
| + | # Validate the inspection procedures used | ||
| + | |- | ||
| + | !rowspan="2"|Design Output Documents | ||
| + | | Completeness of drawings | ||
| | | | ||
| + | # Check if the CAD diagrams overlay and "fit" perfectly | ||
| + | # Check tolerance stackups | ||
| + | |rowspan="2"| Quality systems, CAD | ||
| + | |- | ||
| + | | Correlate First Article Inspection data to the dimensions on the drawings | ||
| + | | | ||
| + | # Obtain First Article Inspection data | ||
| + | # Check if this data correlates with the completeness of drawings | ||
| + | |- | ||
| + | !rowspan="4"|Manufacturing Documents | ||
| + | | Manufacturing procedures, component specifications, raw material specifications, incoming and in-process inspection procedures for completeness | ||
| + | | | ||
| + | # Verify the Bill of Materials corresponds to raw materials and manufacturing procedures | ||
| + | # Correlate incoming and in-process inspection procedures with the process specifications | ||
| + | | rowspan="4"|Material science, manufacturing engineering, quality systems | ||
| + | |- | ||
| + | | Linkage between component and raw material specifications and appropriate incoming inspection procedures | ||
| + | | | ||
| + | # Identify any missing documentation for inspection procedures | ||
| + | |- | ||
| + | |- | ||
| + | | Inspection procedures have adequate sampling plans based on PFMEA risk mitigation levels – this includes packaging and labeling materials | ||
| + | | | ||
| + | # Review supplier audit reports for compliance | ||
| + | |- | ||
| + | | Calibration records and preventive maintenance records; in-process / incoming inspection test methods and related test method validations | ||
| + | | | ||
| + | # Check the entire equipment-related lifecycle | ||
| + | # Check if machine operational qualification was performed | ||
| + | # Check if the measurement equipment was validated | ||
| + | |- | ||
| + | !rowspan="2"|Validation Report | ||
| + | | DV&V reports, Shelf-life reports, Biocompatibility test reports, Sterilization reports, Packaging Validation reports, Process Validation Reports | ||
| | | | ||
| + | # Ensure all reports are available and linked together appropriately | ||
| + | # Identify all inconsistencies across different reports | ||
| + | | rowspan="2"| Quality systems | ||
| + | |- | ||
| + | | Design test methods and related test method validations | ||
| + | | | ||
| + | # Compare test methods used to those in client and ISO standards | ||
| + | # Identify inconsistencies across test methods | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | === Sample report === | ||
| + | ==== Performance/Functional Characteristics ==== | ||
| + | {| class="wikitable" style="font-size:90%" border="1" cellpadding="5" cellspacing="0" | ||
| + | |- style="background:lightgrey" | ||
| + | !align = "center" bgcolor = "#00CCFF" colspan = "4" |Design Input | ||
| + | !align = "center" bgcolor = "#00CCFF" width="5%" rowspan="2"|Design Output | ||
| + | !align = "center" bgcolor = "#00CCFF" width="5%" rowspan="2"|Design Verification Report # | ||
| + | !align = "center" bgcolor = "#00CCFF" width="5%" rowspan="2"|Status (P/F/R) | ||
| + | !align = "center" bgcolor = "#00CCFF" width="5%" rowspan="2"|Design Validation Report # | ||
| + | !align = "center" bgcolor = "#00CCFF" width="5%" rowspan="2"|Status (P/F/R) | ||
| + | |- | ||
| + | !align = "center" bgcolor = "#00CCFF" width="20%"|User Needs | ||
| + | !align = "center" bgcolor = "#00CCFF" width="15%"|User Need Rationale | ||
| + | !align = "center" bgcolor = "#00CCFF" width="20%"|Engineering Specification | ||
| + | !align = "center" bgcolor = "#00CCFF" width="20%"|Engineering Specification Rationale | ||
| + | |- | ||
| + | |Provide antimicrobial resistance for up to 2 weeks | ||
| + | |Ureteral Stent User Survey (Document #XXXXX) | ||
| + | |Stent must have chlorohexadine surface concentration of 10-20 mg/cm2 for 3 weeks | ||
| + | |Document #XXXXX | ||
| + | |Test Document #XXXXX | ||
| + | |Report 01-005-06-007 | ||
| + | |P | ||
| + | |Report 01-005-06-007 | ||
| + | |P | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | === Potential DHF Review Outcomes === | ||
| + | Based on a review of the above DHF documents a potential outcome for the uretral stent acquisition project could involve the following: | ||
| + | # Better explanation of existing design input documents and also better linkage between the design inputs and product specifications. | ||
| + | # Creation of some new test methods for design, incoming and in-process inspections and also include recommendations for the test method validations. Creation of any new DV&V data would be highly unlikely as it could potentially trigger a new submission or a note-to-file to the regulatory agencies. | ||
| + | # Change in raw materials to better grade materials e.g. Switching resin to a USP Class VI biocompatible resin. This would eliminate some on-going testing but require additional upfront one time biocompatibility testing. | ||
| + | # Updating drawings based on results from the FAI data. | ||
| + | # Converting existing Company Y documents into Company X format and identifying potential gaps and streamlining linkage between raw material specifications and inspection procedures. | ||
| + | # Identifying installation, operational and process qualification requirements with the assumption that no additional design verification and validation activities are required based on the fact that the device is currently approved for sale in the US and ROW. | ||
| + | # Recommend activities necessary for completing packaging, labeling, ship testing and shelf-life testing. Stress should be on being able to leverage existing data for shelf-life without changing the regulatory status of the device. | ||
| + | # Company X may want to perform additional biocompatibility testing to create an internal baseline and also update their biocompatibility files. | ||
| + | # Help streamline suppliers for components when switching over from Company Y to Company X. Search for existing Company X suppliers that can supply off the shelf items that Company Y may be sourcing from other vendors / suppliers. | ||
| + | # Identify process improvements that can be rolled into the manufacturing transfer without changing the design and impacting the existing regulatory status for the device e.g. instead of hand mixing pigment to resin use a pre-mixer to control quality of mixing and resulting extrusion or perform the molding and over-molding steps in 1 machine instead of 2 separate molding machines. | ||
| + | |||
| + | == Phase 3: Post-acquisition integration == | ||
| + | === Deadlines === | ||
| + | '''Goal''': Switch production transparently to new facilities transparently to the distribution system | ||
| + | {| class="wikitable" style="font-size:90%" border="1" cellpadding="5" cellspacing="0" | ||
| + | |- style="background:lightgrey" | ||
| + | !align = "center" bgcolor = "#00CCFF" |Stage | ||
| + | !align = "center" bgcolor = "#00CCFF" |Tasks | ||
| + | !align = "center" bgcolor = "#00CCFF" |Milestone payment | ||
| + | !align = "center" bgcolor = "#00CCFF" |Date | ||
|- | |- | ||
| − | | | + | |Design center integration plan |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| | | | ||
| + | * Gap analysis completion (acquiree) | ||
| + | * Gap analysis completion (acquirer) | ||
| | | | ||
| − | | | + | |September 15, 2008 |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | | | + | |Design to manufacturing transfer |
| | | | ||
| − | |||
| | | | ||
| − | | | + | |December 15, 2008 |
| − | | | + | |- |
| + | |Equipment transfer | ||
| | | | ||
| + | |Milestone I payment | ||
| + | |Jan 7, 2009 | ||
| + | |- | ||
| + | |Shut production at acquiree facility | ||
| + | |Negotiation for contract extension | ||
| + | |Milestone II payment | ||
| + | |Feb 15, 2009 | ||
| + | |- | ||
| + | |Start production in acquirer facility | ||
| | | | ||
| − | |||
| | | | ||
| + | |Feb 7, 2009 | ||
| + | |- | ||
| + | |Switch to new SKU | ||
| | | | ||
| | | | ||
| + | |Feb 15, 2009 | ||
| + | |- | ||
| + | |End development of new generation product/s in old facility | ||
| | | | ||
| | | | ||
| + | |Feb 7, 2009 | ||
| + | |- | ||
| + | |Restart development of new generation product/s post-acquisition | ||
| + | | | ||
| + | |Final milestone payment | ||
| + | |Mar 1, 2009 | ||
| + | |- | ||
|} | |} | ||
| − | == | + | === Documents and Ownership === |
| − | + | {| class="wikitable" style="font-size:90%" border="1" cellpadding="5" cellspacing="0" | |
| − | + | |- style="background:lightgrey" | |
| − | + | !align = "center" bgcolor = "#00CCFF" |Document | |
| − | {|border=" | + | !align = "center" bgcolor = "#00CCFF" |Owner |
| − | | | + | !align = "center" bgcolor = "#00CCFF" |Last update date |
| − | | | + | |- |
| + | |Product performance specifications | ||
| + | |Paul Swain | ||
| + | |07/27/2008 08:15:35 PST | ||
| + | |- | ||
| + | |Component specifications | ||
| + | |Kevin Teller | ||
| + | |06/12/2008 12:22:07 PST | ||
| + | |- | ||
| + | |Preclinical test results | ||
| + | |Joanne Krannert | ||
| + | |07/03/2008 14:17:00 PST | ||
| + | |- | ||
| + | |Clinical tests | ||
| + | |Joanne Krannert | ||
| + | |08/01/2008 08:00:55 PST | ||
|- | |- | ||
|} | |} | ||
| − | |||
| + | ==Ureteral stents regulatory issues== | ||
| + | The FDA classifies a ureteric stent as follows: | ||
| + | * TITLE 21 - FOOD AND DRUGS | ||
| + | * CHAPTER I - FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES | ||
| + | * SUBCHAPTER H - MEDICAL DEVICES | ||
| + | * PART 876 - GASTROENTEROLOGY-UROLOGY DEVICES | ||
| + | * Subpart E - Surgical Devices | ||
| + | * Sec. 876.4620 - Ureteral stent. | ||
| + | * Classification - class II device [http://www.accessdata.fda.gov/SCRIPTS/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=876.4620&SearchTerm=ureter%20stent Code of Federal Regulations] | ||
| + | ===Pre-Market Notification=== | ||
| + | Section 510(k) of the Food, Drug and Cosmetic Act requires device manufacturers who must register, to notify FDA of their intent to market a medical device at least 90 days in advance, also known as Premarket Notification. This premarket submission demonstrates to the FDA that the device to be marketed is atleast as safe and effective, that is, ''substantially equivalent'', to a legally marketed device. Parties required to submit a 510(k) to the FDA include domestic or foreign manufacturers introducing a device to the U.S. market, as well as specification developers and repackers/relabelers. | ||
| − | + | A 510(k) is required when: | |
| − | + | * Introducing a device into commercial distribution (marketing) for the first time. | |
| − | * | + | * Proposed different intended use for a device already in commercial distribution. |
| − | + | * Change or modification of a legally marketed device. | |
| − | === | + | [http://dolcera.com/upload/files/510kflowchart.pdf 510(k) “Substantial Equivalence” Decision Making Process] |
| − | + | ||
| + | Some of the companies active in the field of ureteral stents have been represented in the table below. (This is not an exhaustive list and is just a sample) | ||
| + | |||
| + | {| border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | | style="background-color:#ffcc99;padding:0.079cm;"| <center>'''Sr. No. '''</center> | ||
| + | | style="background-color:#ffcc99;padding:0.079cm;"| <center>'''Company'''</center> | ||
| + | | style="background-color:#ffcc99;padding:0.079cm;"| <center>'''Device'''</center> | ||
| + | | style="background-color:#ffcc99;padding:0.079cm;"| <center>'''Approval'''</center> | ||
| + | | style="background-color:#ffcc99;padding:0.079cm;"| <center>'''Date of Approval'''</center> | ||
| + | | style="background-color:#ffcc99;padding:0.079cm;"| <center>'''Material'''</center> | ||
| + | | style="background-color:#ffcc99;padding:0.079cm;"| <center>'''Technology'''</center> | ||
| + | | style="background-color:#ffcc99;padding:0.079cm;"| <center>'''Indwelling time (days) '''</center> | ||
| + | | style="background-color:#ffcc99;padding:0.079cm;"| <center>'''Image'''</center> | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#ffcc99;padding:0.079cm;"| <center>'''1'''</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| <center>[http://www.bardurological.com/products/categoryTwo.aspx?bUnitID=3&catOneID=71 Bard Urological]</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| <center>[http://www.bardurological.com/products/loadProduct.aspx?bUnitID=3∏ID=225 InLay Optima]</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| <center>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=16869 FDA 510(k)]</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| <center>Dec 2004</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| Silicone | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| Double pigtail with monofilament suture loop | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| <center>365</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| [[Image:InLay_Optima.png|thumb|center|200px|<center>InLay Optima</center>]] | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#ffcc99;padding:0.079cm;"| <center>'''2'''</center> | ||
| + | | style="padding:0.079cm;"| <center>[http://www.bostonscientific.com/Device.bsci/,,/method/DevHome/navRelId/1000.1003/seo.serve Boston Scientific]</center> | ||
| + | | style="padding:0.079cm;"| <center>[http://www.bostonscientific.com/urology-stone/product.html?method=product_detail∏uct_id=10122561#initialLoad1() Polaris Loop]</center> | ||
| + | | style="padding:0.079cm;"| <center>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=10929 FDA 510(k)]</center> | ||
| + | | style="padding:0.079cm;"| <center>Mar 2003</center> | ||
| + | | style="padding:0.079cm;"| Dual Durometer Percuflex with HydroPlus Coating | ||
| + | | style="padding:0.079cm;"| Bladder loop design | ||
| + | | style="padding:0.079cm;"| <center>365</center> | ||
| + | | style="padding:0.079cm;"| [[Image:Polaris_Loop.png|thumb|center|200px|<center>Polaris Loop</center>]] | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#ffcc99;padding:0.079cm;"| <center>'''3'''</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| <center>[http://www.cookmedical.com/uro/familyListingAction.do?family=Ureteral+Stents Cook Medical]</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| <center>[http://www.cookmedical.com/uro/dataSheet.do?id=4418 Resonance]</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| <center>[http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=23620 FDA 510(k)]</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| <center>May 2007</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| Metal | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| Temporary stenting | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| <center>365</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| [[Image:Resonance.png|thumb|center|200px|<center>Resonance</center>]] | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#ffcc99;padding:0.079cm;" rowspan="2"| <center>'''4'''</center> | ||
| + | | style="padding:0.079cm;" rowspan="2"| <center>[http://www.fossamedical.com/news.htm Fossa Medical]</center> | ||
| + | | style="padding:0.079cm;" rowspan="2"| <center>[http://dolcera.com/upload/files/stonesweeper_fossa_trial.pdf Stone Sweeper]</center> | ||
| + | | style="padding:0.079cm;"| <center>[http://www.fossamedical.com/news.htm FDA 510(k)]</center> | ||
| + | | style="padding:0.079cm;"| <center>Aug 2002</center> | ||
| + | | style="padding:0.079cm;" rowspan="2"| Polyurethane | ||
| + | | style="padding:0.079cm;" rowspan="2"| Spiral radially expanding stent | ||
| + | | style="padding:0.079cm;" rowspan="2"| <center>13</center> | ||
| + | | style="padding:0.079cm;" rowspan="2"| [[Image:Stone_Sweeper.png|thumb|center|200px|<center>Stone Sweeper</center>]] | ||
| + | |||
| + | |- | ||
| + | | style="padding:0.079cm;"| <center>[http://www.fossamedical.com/news.htm CE Mark]</center> | ||
| + | | style="padding:0.079cm;"| <center>Sep 2005</center> | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#ffcc99;padding:0.079cm;"| <center>'''5'''</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| <center>[http://www.pnnmedical.com/urology/professionals/products/memokath™-051-ureter.aspx Pnn Medical A/S]</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| <center>[http://www.google.com/url?sa=t&source=web&cd=4&ved=0CC4QFjAD&url=http://www.hammer.pl/pliki/147_2.pdf&rct=j&q=memokath%20051&ei=MfhATezNIoaqvQP-_ZGtAw&usg=AFQjCNFR-ZFsu33rk6B9Flq1tCsYBZyXMw&cad=rja Memokath 051]</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| <center>[http://www.pnnmedical.com/about-pnn-medical/company-history.aspx CE Mark]</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| <center>1995</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| Nickel-titanium shape memory alloy | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| Double fluted ended spiral stent | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| <center>240</center> | ||
| + | | style="background-color:#ccffff;padding:0.079cm;"| [[Image:Memokath_051.png|thumb|center|200px|<center>Memokath 051</center>]] | ||
| + | |||
| + | |} | ||
| − | [[ | + | ===Timeline Sheet=== |
| + | [[Media:Ureteral_Stents_Timeline dw.xls|Ureteral Stent Timeline]] | ||
| − | |||
| − | |||
| − | |||
| − | + | *References | |
| − | * | + | |
| − | + | ||
| − | + | 1- [http://en.wikipedia.org/wiki/Demographics_of_the_United_States http://en.wikipedia.org/wiki/Demographics_of_the_United_States] | |
| − | + | ||
| − | = | + | 2- [http://www.wrongdiagnosis.com/c/catheter_infection/stats.htm?ktrack=kcplink http://www.wrongdiagnosis.com/c/catheter_infection/stats.htm?ktrack=kcplink] |
| − | + | ||
| − | [ | + | 3- [http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/WomensHealthResearch/UCM247851.pdf http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/WomensHealthResearch/UCM247851.pdf] |
| − | + | 4- [http://www.managementparadise.com/forums/principles-management-p-o-m/208329-swot-analysis-boston-scientific-corporation.html http://www.managementparadise.com/forums/principles-management-p-o-m/208329-swot-analysis-boston-scientific-corporation.html] | |
| − | + | ||
| − | =<span style="color:#C41E3A">Like this report?</span>= | + | ==<span style="color:#C41E3A">Like this report?</span>== |
<p align="center"> '''This is only a sample report with brief analysis''' <br> | <p align="center"> '''This is only a sample report with brief analysis''' <br> | ||
'''Dolcera can provide a comprehensive report customized to your needs'''</p> | '''Dolcera can provide a comprehensive report customized to your needs'''</p> | ||
| Line 1,130: | Line 1,041: | ||
|} | |} | ||
<br> | <br> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | ==Contact Dolcera== | |
| − | + | ||
| − | + | ||
| − | =Contact Dolcera= | + | |
{| style="border:1px solid #AAA; background:#E9E9E9" align="center" | {| style="border:1px solid #AAA; background:#E9E9E9" align="center" | ||
| Line 1,194: | Line 1,053: | ||
|} | |} | ||
| − | [[ | + | = Addition = |
| + | === Patent Categorization: Interactive mind map linked to Dolcera Dashboard === | ||
| + | * To access the Dashboard you have to signup. You can do so by clicking [https://www.dolcera.com/auth/index.php/login '''here'''] | ||
| + | *''Use the mouse(click and drag/scroll up or down/click on nodes) to explore nodes in the detailed taxonomy'' | ||
| + | *''Click on the red arrow adjacent to the node name to view the content for that particular node in the dashboard'' | ||
| + | *''Click on the "+" sign to zoom the mindmap and "-" sign to shrink the mindmap'' | ||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | |||
| + | |<mm>[[Ureteral_Stent_Patent_Categorization.mm|flash|Patent Categorization|600pt]]</mm> | ||
| + | |||
| + | |} | ||
| + | |||
| + | |||
| + | === Product-Patent-Clinical Trials Mapping === | ||
| + | * To access the Dashboard you have to signup. You can do so by clicking [https://www.dolcera.com/auth/index.php/login '''here'''] | ||
| + | *''Use the mouse(click and drag/scroll up or down/click on nodes) to explore nodes in the detailed taxonomy'' | ||
| + | *''Click on the red arrow adjacent to the node name to view the content for that particular node in the dashboard'' | ||
| + | *''Click on the "+" sign to zoom the mindmap and "-" sign to shrink the mindmap'' | ||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | |||
| + | |<mm>[[ureteral_stent_mapping1.mm|flash|Ureteral Stent mindmap|600pt]]</mm> | ||
| + | |||
| + | |} | ||
| + | |||
| + | === Product to Patent Mapping === | ||
| + | [[Image:Product_Patent_Mapping_Screen_Shot.png|1000px|centre|thumb|'''Screenshot for the product to patent mapping(Bard and Boston)''']] | ||
| + | * Click [[Media:Product_Patent_Mapping_Bard_Boston.xls|'''here ''']]to download the excel file. | ||
| + | * Mapped Patent vs Not Mapped Patents | ||
| + | {|border="0" cellspacing="0" cellpadding="4" width="100%" | ||
| + | |[[Image:CRB_Pat.png|center|500px|thumb|'''C R Bard''']] | ||
| + | |[[Image:BS_pat.png.png|center|500px|thumb|'''Boston Scientific''']] | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | === Dolcera Dashboard === | ||
| + | [[Image:dashboard_features.png|center|750px|]] | ||
| + | |||
| + | '''Dashboard Link'''<br> | ||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | |'''[http://client.dolcera.com/dashboard/dashboard.html?workfile_id=1008 Ureteral Stent - Dashboard] ''' | ||
| + | |width="100"|[[Image:dashboard_thumb.png|center|100px|]] | ||
| + | |- | ||
| + | |} | ||
| + | *Flash Player is essential to view the Dolcera Dashboard | ||
| + | * To access the Dashboard you have to signup. You can do so by clicking [https://www.dolcera.com/auth/index.php/login '''here'''] | ||
| + | |||
| + | = Key Artifacts = | ||
| + | * [[Investment Heat Map ]] | ||
| + | * [[Revenue Heat Map ]] | ||
| + | * [[Patent Heat Map]] | ||
| + | * [http://www.dolcera.com/website/demos/dental/main.html Dynamic Patent Dashboard] | ||
| + | * [http://www.dolcera.com/ipmapdemo/stent_model.swf Stent Landscape-Flash] | ||
| + | * [[Company Profile - Flash]] | ||
| + | |||
| + | =Removed Sections= | ||
| + | [[Removed Sections]] | ||
Revision as of 05:45, 16 February 2012
Contents
- 1 Process to Identify Possible Acquisition Targets
- 2 M&A Due Diligence Process
- 3 Phase 1: Landscape overview
- 4 Urinary Problems in men and women
- 5 Market Analysis
- 6 Like this report?
- 7 Phase 2: Deeper Dive
- 8 Phase 3: Post-acquisition integration
- 9 Ureteral stents regulatory issues
- 10 Like this report?
- 11 Contact Dolcera
- 12 Addition
- 13 Key Artifacts
- 14 Removed Sections
Process to Identify Possible Acquisition Targets
Before identifying the possible acquisition targets, first companies needs to identify the objective of the merger and acquisition.
Advantage of Mergers and Acquisitions
The most common motives and advantages of mergers and acquisitions are:-
- Accelerating a company's growth, particularly when its internal growth is constrained due to paucity of resources. Internal growth requires that a company should develop its operating facilities- manufacturing, research, marketing, etc. But, lack or inadequacy of resources and time needed for internal development may constrain a company's pace of growth. Hence, a company can acquire production facilities as well as other resources from outside through mergers and acquisitions. Specially, for entering in new products/markets, the company may lack technical skills and may require special marketing skills and a wide distribution network to access different segments of markets. The company can acquire existing company or companies with requisite infrastructure and skills and grow quickly.
- Enhancing profitability because a combination of two or more companies may result in more than average profitability due to cost reduction and efficient utilization of resources. This may happen because of:-
- Economies of scale:- arise when increase in the volume of production leads to a reduction in the cost of production per unit. This is because, with merger, fixed costs are distributed over a large volume of production causing the unit cost of production to decline. Economies of scale may also arise from other indivisibilities such as production facilities, management functions and management resources and systems. This is because a given function, facility or resource is utilized for a large scale of operations by the combined firm.
- Operating economies:- arise because, a combination of two or more firms may result in cost reduction due to operating economies. In other words, a combined firm may avoid or reduce over-lapping functions and consolidate its management functions such as manufacturing, marketing, R&D and thus reduce operating costs. For example, a combined firm may eliminate duplicate channels of distribution, or crate a centralized training center, or introduce an integrated planning and control system.
- Synergy:- implies a situation where the combined firm is more valuable than the sum of the individual combining firms. It refers to benefits other than those related to economies of scale. Operating economies are one form of synergy benefits. But apart from operating economies, synergy may also arise from enhanced managerial capabilities, creativity, innovativeness, R&D and market coverage capacity due to the complementarity of resources and skills and a widened horizon of opportunities.
- Diversifying the risks of the company, particularly when it acquires those businesses whose income streams are not correlated. Diversification implies growth through the combination of firms in unrelated businesses. It results in reduction of total risks through substantial reduction of cyclicality of operations. The combination of management and other systems strengthen the capacity of the combined firm to withstand the severity of the unforeseen economic factors which could otherwise endanger the survival of the individual companies.
- A merger may result in financial synergy and benefits for the firm in many ways:-
- By eliminating financial constraints
- By enhancing debt capacity. This is because a merger of two companies can bring stability of cash flows which in turn reduces the risk of insolvency and enhances the capacity of the new entity to service a larger amount of debt
- By lowering the financial costs. This is because due to financial stability, the merged firm is able to borrow at a lower rate of interest.
- Limiting the severity of competition by increasing the company's market power. A merger can increase the market share of the merged firm. This improves the profitability of the firm due to economies of scale. The bargaining power of the firm vis-à-vis labour, suppliers and buyers is also enhanced. The merged firm can exploit technological breakthroughs against obsolescence and price wars.
Note: In this research report, researcher considered two following objective of merger and acquisition:
- To Enter in emerging markets
- To improve product portfolio
Methodology
- Step 1: First, list all the players present in Ureteral Stent Market were identified.(The list of companies was retrieved from the FDA site from Registration & Listing database)
- Number of companies that were identified in this step: 20
- Step 2: The second step involved identifying and eliminating companies that are large, and established players or the subsidiaries of the big players in the industry.
- Number of companies eliminated in this step: 14
- Number of (small) companies of interest left: 6
- Step 3: Once the large, and established players were eliminated, companies were compared based on various parameters and rated on the scale of 5 to identify the best target. Please check the following table and dashboard:
- Number of potential target companies: 6
| Company | Headquartered | Stent Details | Company Type | 2010 Revenue (Mn) | No of Employees | Geographical Revenue Share | R&D investment (Mn) | No. of Patent | Technology Focus |
| Applied Medical Resources Corp. | USA | Mesh ureteral stent C-flex ureteral stent Silicone ureteral stent Tethered ureteral stent C-flex ureteral stent Ureteral stent Silhouette pediatric stent |
Manufacturer | $282 | 1900 | USA: 62% Asia: 32% Rest of World: 6% |
$38.3 (13%) | 212 | High |
| Bioteque Corp. | Taiwan | Bioteque Double Pigta | Contract Manufacturer; Contract Sterilizer; Manufacturer | $27.85 | 350 | Taiwan: 100% | $1.4 (5%) | 16 | Medium |
| Hobbs Medical, Inc. | USA | HM Ureteral Double Pigtail Stent | Manufacturer | $2.30 | 22 | USA: 97.2% Japan: 2.3% Others: 0.5% |
$0.06 (2.87%) | 1 | Low |
| Lake Region Medical Limited | Ireland | M-Wires | Contract Manufacturer | $84.86 | 611 | Europe: 68% USA: 21% Others: 11% |
$8.48 (3.85%) | 8 | Medium |
| Martech Medical Products | USA | Ureteral Stent | Contract Manufacturer | $23.00 | 180 | USA: 78.3% Europe: 12.7% Others: 9% |
$1.1 (4.79%) | 0 | Low |
| Allium Medical | Israel | URS - Ureteral Stent TPS - Triangular Prostatic Stent BUS - Bulbar Urethral Stent RPS - Round Posterior Urethral Stent BIS - Biliary Stent |
Manufaturer | $2.04 | 20 | Domestic: 88.6% International: 11.4% |
$0.082 (4%) | 2 | Low |
- Step 4: Finally, after an in-depth analysis of the potential target companies’ on various parameters, following companies identified to be best possible target companies.
- To improve Product Portfolio : Applied Medical Resources Corp.
- To enter in Emerging Markets : Bioteque Corp.
Company Profile
Applied Medical Resources Corp.
| APPLIED MEDICAL RESOURCES CORP. | |
| Revenues | 2010: $280 million |
| Net profit (2010) | $20.89 million |
| R&D Investment | 2010: $ 38.3 million (13% of revenues) |
| Number of employees | 1900 |
| Year Established | 1987 |
| Headquarters | USA |
| Key People | CEO: Said S. Hilal |
| Products & Technology | Specialty Areas:Cardiac/Vascular, Colorectal, GYN, Urology New Products:Epix (Laparoscopic Intrumentation), GelPOINT (Advanced Access Platform), Kii Fios (First EntrySystem) |
| Products in Ureteral Stent | C-flex ureteral stent, Silicone ureteral stent, Tethered ureteral stent, C-flex ureteral stent, Ureteral stent Silhouette pediatric stent |
| Geographical revenue breakdown (2010) | USA: $182.70 million (65%) Others: $99.47 million (35%) |
| Company Overview | Applied Medical Resources Corporation is a new generation medical device company founded in 1987 and headquartered in Southern California. It is involved in developing, manufacturing and marketing of innovative products for Minimally Invasive Surgery, Cardiac, Vascular, Urological, Colorectal, Bariatric, Obstetric, Gynecologic and General Surgery. The product portfolio covers 25 technologies and more than 700 products. The company has spread its business globally across 75 countries including Africa, Middle East,Americas, Caribbean, Asia, Australia and Europe through its network of international distributors. |
Bioteque Corp.
| Bioteque Corp. | |
| Revenues | 2010: $27.85 million |
| Net profit (2010) | $20.89 million |
| R&D Investment | 2010: $38.3 million (13% of revenues) |
| Number of employees | 350 |
| Year Established | 1991 |
| Headquarters | Taiwan |
| Key People | |
| Products & Technology | NEPHROLOGY, UROLOGY, RADIOLOGY, CARDIOLOGY, RESPIRATORY CARE CRITICAL CARE, IV ADMINISTRATION THERAPY, MOLDING PARTS |
| Products in Ureteral Stent | C-flex ureteral stent, Silicone ureteral stent, Tethered ureteral stent, C-flex ureteral stent, Ureteral stent Silhouette pediatric stent |
| Geographical revenue breakdown (2010) | NA |
| Company Overview | Bioteque Corporation manufactures and sells medical devices in Taiwan. It offers medical disposables for use in hemodialysis access, endovascular treatment, and other fields. It offers blood tubing lines, AVF needles, transducer protectors, and on line HDF with check valves or without check valves; IV infusion bags, precision IV infusion sets, drainage bags, insufflation tubing sets/filters, and various surgical drainage tubes; a range of medical components, which comprise blood tubing line components, percutaneous drainage components, infusion bag components, AVF needle components, precision IV infusion set components, and IV infusion bag components; a range of thermoplastic polyurethane catheters, including pigtail drainage catheter sets, double pigtail ureteral stent sets, biliary drainage catheters, percutaneous nephrostomy kits, and dialysis catheters; and other medical disposable products, such as closed suction catheters and artificial nose. The company also provides endovascular products, which consist of percutaneous transluminal coronary angioplasty, percutaneous transluminal angioplasty, angiography catheters, guiding catheters, sheath introducers, MRI/CT/angiography syringes, micro catheters, and hydrophilic coated guidewires. Bioteque Corporation was founded in 1991 and is based in Taipei, Taiwan. |
M&A Due Diligence Process
Phase 1: Landscape overview
Ureteral Stent: Concept
An antimicrobial ureteral stent, which inhibits encrustation and bacterial colonization while maintaining patient comfort.
- Ureteral stent: resists migration, resists fragmentation, is kink resistant and radiopaque.
- Bacterial colonization: antimicrobial activity for up to two weeks.
- Patient Comfort: stent has a low coefficient of fiiction (value) for ease of insertion and will soften on implant at body temperature to maintain patient comfort.
Background
Ureteral stents are used in urological surgery to maintain patency of the ureter to allow urine drainage from the renal pelvis to the bladder. These devices can be placed by a number of different endourological techniques. They are typically inserted through a cystoscope and may also be inserted intraoperatively. Indwelling ureteral stents help to reduce complications and morbidity subsequent to urological and surgical procedures. Frequently, ureteral stents are used to facilitate drainage in conjunction with Extracorporeal Shock Wave Lithotripsy (ESWL) and after endoscopic procedures. They are also used to internally support anastomoses and prevent urine leakage after surgery. Ureteral stenting may almost eliminate the urological complications of renal transplantation.
The advent of ESWL and the more recent barrage of endourological techniques have increased the indications for ureteral stents (Candela and Bellman 1997). Indications for use include:
- Treatment of ureteral or kidney stones
- Ureteral trauma or stricture
- Genitourinary reconstructive surgery
- Hydronephrosis during pregnancy
- Obstruction due to malignancy
- Retroperitoneal fibrosis
The need for ureteral stents range from a few days to several months. For patients with serious urological problems, ureteral stent maintenance may become a life-long necessity. Unfortunately, there are many problems associated with using ureteral stents.
Ureteric stenting difficulties
| Common | Rare |
|
|
Today, elastomeric materials, such as silicones, polyurethanes and hydrogel-coated polyolefins are used, with no clear winner, which can withstand the urinary environment.
- Although silicone has better long-term stability than other stent materials, its extreme flexibility makes it difficult to pass over guidewires and through narrow or tortuous ureters.
- Polyethylene is stiffer and easier to use for patients with strictures; however, it has been known to become brittle with time leading to breakage and is no longer commercially available. * Polyurethane has properties that fall in between polyethylene and silicone; however, stent fracture also has been an issue with polyurethanes.
Attempts have been made to develop polymers with a combination of the best of all properties. The key players are C-Flex (Concept Polymer Technologies), Silitek and Percuflex (Boston Scientific).
- C-Flex is proprietary silicone oil and mineral oil interpenetrated into a styrenelolefin block copolymer with the hope of reduced encrustation.
- Silitek (Medical Engineering Corporation) is another silicone-based copolymer.
- Percuflex is a proprietary olefinic block copolymer.
Metallic stents have been used recently to treat extrinsic ureteric obstructions. The effect of synthetic polymers on the urothelium of the urinary tract seems to be dependent on the bulk chemical composition of the polymer, the chemical composition of its surface, coatings on the device surface, smoothness of the surface and coefficient of friction.
Typically, most ureteral stents are made of relatively smooth catheters. Koleski et al., (2000) tested a longitudinally grooved ureteral stent made by Circon in the pig ureter. The results indicated that the grooved stent led to better drainage than a conventional stent. Their opinion is that the ureter wall has a better chance of collapsing over a smooth surface than a grooved surface, especially when debris is present. Stoller (2000) had the same experience with the SpiraStent(Urosurge Corp.). This helical stent was superior at passing stones than a conventional smooth stent.
There are a variety of ureteral stent configurations with different anchoring systems. Most stents today have a double pigtail anchoring system. (Tolley, 2000), Dunn et al, (2000) conducted a randomized, single-blind study comparing a Tail stent (proximal pigtail with a shaft which tapers to a lumenless straight tail) to a double pigtail stent. The Tail stent was found to be better tolerated than the double-pigtail concerning lower urinary tract irritative symptoms. A double-J ureteral stent and a flexible ureteropyeloscope are shown in the first diagram. The other two diagrams show a pigtail ureteral stent in place; the end of the pigtail is facing away fiom the ureteral opening in the second of these two diagrams.
Early adverse effects of ureteral stenting include lower abdominal pain, dysuria, fever, urinary frequency, nocturia and hematuria. Patient discomfort and microscopic hematuria happen often. Major late complications include stent migration, stent fragmentation or more serious hydronephrosis with flank pain and infections.
Late complications occurred in one third of the patients in a prospective study using both silicone and polyurethane double pigtail stents (110 stents) in 90 patients. Stent removal was necessary in these patients. Others also have found this percentage of late complications. Device-related urinary tract infection and encrustation can lead to significant morbidity and even death and are the primary factors limiting long-term use of indwelling devices in the urinary tract. Microbial biofilm and encrustation may lead to stone formation. This is typically not a problem when stents are used for short-term indications. Problems of biofilm formation, encrustation and stent fracture occur in patients with long-term indwelling stents.
Typically, manufacturers advise periodic stent evaluation. Cook polyurethane stent removal is recommend at 6 months and 12 months for silicone (Cook product literature). However, stents that are intended for long-term use are usually changed at regular intervals, as frequently as every 3 months.
Forgotten stents are a problem. Monga et al., 1995 found that 68% of stents forgotten more than 6 months were calcified and 10% were fragmented. Multiple urologic procedures were necessary to remove the stones. Long-term effects of these forgotten stents may lead to voiding dysfunction and renal insufficiency. Schlick, et al., 1998 are developing a biodegradable stent that will preclude the need for stent removal.
Encrustation
The urinary system presents a challenge because of its chemically unstable environment. Long-term biocompatibility and biodurability of devices have been problems due to the supersaturation of uromucoids and crystalloids at the interface between urine and the device. Encrustation of ureteral stents is a well-known problem, which can be treated easily if recognized early. However, severe encrustation leads to renal failure and is difficult to manage (Mohan-Pillai et al., 1999). All biomaterials currently used become encrusted to some extent when exposed to urine.
The encrusted deposits can harbor bacterial biofilms. In addition, they can render the biomaterial brittle which causes fracture in-situ, a serious problem especially associated with the use of polyethylene and polyurethane ureteral stents (although silicone stents have also been reported to fracture). Stent fragments can migrate to the bladder or renal pelvis with serious repercussions.
Surface science techniques were used to study three stent types after use in patients. The stent type, duration of insertion and age or sex of the patient did not correlate significantly with the amount of encrustation (Wollin et al., 1998). However, it has been suggested that factors which affect the amount of encrustation include the composition or the urine, the type of invading and colonizing bacteria and the structure and surface properties of the biomaterial used (Gorman 1995). A low surface energy surface seems to resist encrustation compared with a high surface energy surface (Denstedt et al., 1998).
Many different types of stone can form in the urinary tract. Calcium oxalate, calcium phosphate, uric acid and cystine stones are metabolic stones because they form as a result of metabolic dysfunction. They usually are excreted from the urinary tract. Struvite (magnesium ammonium phosphate) and hydroxyapatite (calcium phosphate) are associated with infection (infection stones). These account for 1520% of urinary calculi. ESWL is used to break up the larger infection stones because they don't pass; recurrence of the problem occurs with incomplete removal. Infection stones can manifest as poorly mineralized matrix stones, highly mineralized staghorn calculi or as bladder stones which often form in the presence of ureteral stents. Urea-splitting bacteria colonize the surface and cause alkalinization of the urine, which lowers the solubility of struvite and hydroxyapatite, and they deposit on the surface. Bacterial biofilm associated with encrustation is a common clinical occurrence. (Gorman and Tunney, 1997). It has been suggested that prevention of bacterial colonization would prevent encrustation because of their ultimate responsibility for its formation (Bibby et al., 1995).
An in vitro model was developed that produces encrustation similar to those seen in vivo (Tunney et al., 1996a). An experiment was conducted to compare the encrustation potential of various ureteral stent materials. The long-term struvite and hydroxyapatite encrustation of silicone, polyurethane, hydrogel-coated polyurethane, Silitek and Percuflex were compared. All of the materials developed encrustation, however, it was found by image analysis that the rates of encrustation varied on the different materials. Silicone had less encrustation (69% at 10 weeks) compared to the other materials (1 00%) at the same time point (Tunney et al., 1996b). Continuous flow models have also been developed which are more representative of conditions in the upper urinary tract. They are discussed by Gorman and Tunney, (1 997). Efforts to reduce encrustation using new materials, smoother surfaces and hydrogel coatings have been attempted.
A hydrogel-coated C-flex stent (Hydroplus, Boston Scientific) was shown to have less epithelial cell damage and encrustation than other biomaterials and was recommended by the investigators for long-term use (Cormio, 1995). In addition, a poly(ethy1ene oxide)/polyurethane composite hydrogel (Aquavenem, J & J) resisted intraluminal blockage in a urine flow model compared with silicone and polyurethane (Gorman et al., 1997a). Another advantage with Aquavene is that it is rigid in the dry state, which facilitates insertion past obstructions in the ureter and becomes soft on hydration providing comfort (Gorman and Tunney, 1997). Gorman et al. (1997b) concluded that the chance of stent fracture would be reduced if the ureteral stent side holes were eliminated. Urinary tract infection is another common major problem with the usage of ureteral stents. Initially, a conditioning film is deposited on the ureteral stent surface. The film is made up of proteins, electrolyte materials and other unidentified materials that obscure the surface properties of the stent material. Electrostatic interactions, the ionic strength and pH of the urine and differences in fluid surface tensions affect bacterial adhesion to the conditioning film. Subsequently, a microbial biofilm forms over time. The biofilm is composed of bacterial cells embedded in a hydrated, predominantly anionic mixture of bacterial exopolysaccharides and trapped host extracellular macromolecules.
Obstruction
Obstruction of urine flow and urinary tract sepsis can result in continued growth of the biofilm. Colonization of devices implanted in the urinary tract can lead to dysfunction, tissue intolerance, pain, subclinical or overt infection and even urosepsis. Device related infections are difficult to treat and device removal is usually necessary. The biofilm has been found to impede the diffusion of antibiotics; in addition, the bacteria in the biofilm have a decreased metabolic rate , which also protects them against the effects of antibiotics (Wollin et al., 1998). Riedl, et al. (1 999) found 100% ureteral stent colonization rates in permanent and 69.3% in temporary stents. Antibiotic prophylaxis did not prevent bacterial colonization and it was recommended that it not be used. On the other hand, Tieszer, et al. (1 998) believe that fluoroquinolones can prevent infection. They also have found that some stents have denser encrustation than others, however, the stent material did not change the elements of the "conditioning film" adsorbed or alter its receptivity to bacterial biofilms.
Infection
The predictive value of urine cultures in the assessment of stent colonization was examined in 65 patients with indwelling ureteral stents. It was found that a sterile urine culture did not rule out the stent itself being colonized (Lifshitz, et al., 1999). Patients with sterile urine culture may benefit from prophylactic antibiotics; however, the authors contended that the antibiotics must work against gram-negative uropathogens and gram-positive bacteria including enterococci. It is obvious that there is controversy in the literature whether prophylactic systemic antibiotics are useful with ureteral stent implant. However, antibiotics do not seem to prevent stent colonization. Denstedt et al. (1998) have found that ciprofloxacin, with a 3 day burst every 2 weeks, actually is adsorbed onto the stent which makes longer term treatment possible with reduced risk of bacterial resistance. There has been research targeted at coating or impregnating urinary catheters with antimicrobials and products are on the market, however, there are no antimicrobial ureteral stents approved by the FDA.
The market need
It is clear that there is a need for a new material that will be able to resist encrustation and infection in the urinary tract. According to Merrill Lynch, ureteral stents represent an $80 MM US market. Boston Scientific is in the lead with ~50% of the market followed by Maxxim (Circon), Cook and Bard is a smaller player. There are a number of other small contenders.
The use of ureteral stents is increasing; the indications for ureteral stenting have broadened from temporary or permanent relief or ureteric obstruction to include temporary urinary diversion following surgical procedures such as endopyelotomy and ureteroscopy and facilitation of stone clearance after ESWL (Tolley, 2000).
The use of ureteral stents for patients having ESWL for renal calculi is however controversial and seems to be related to the size of the stones and invasiveness of the procedure. According to survey results reported by Hollowell, et al. (2000), there is a significant difference in opinion concerning the use of stents with ESWL.
The number of ureteral stents used in patients with stones 2 cm or less treated with ESWL is significant in spite of the lack scientific evidence in support of this practice. Of 1,029 urologists returning surveys, for patients with renal pelvic stones 10, 15 or 20 rnm treated with ESWL, routine stent placement was preferred by 25.3%, 57.1 % and 87.1 %, respectively. Urologists recommend using ureteroscopy rather than ESWL for distal ureteral calculi 5-1 0 mm.
Intellectual property
Search strategy
- Databases searched: US-G, US-A, EP-A, EP-B, WO, JP, DE, GB, FR
- Search scope: Title, Abstract or Claims
- Years: 1981-July 2008
- Search query: (ureter* OR urether* OR ureth* OR uretr*) AND (stent*) AND (*microb* OR *bacter*)
- Results: 177 patents (82 unique patent families)
Sample patents
| Patent | Assignee | Title | Abstract |
|---|---|---|---|
| US6468649 B1 | SCIMED LIFE SYSTEMS INC | Antimicrobial adhesion surface | The present invention provides an implantable medical device having a substrate with a hydrophilic coating composition to limit in vivo colonization of bacteria and fungi. The hydrophilic coating composition includes a hydrophilic polymer with a molecular weight in the range from about 100, 000 to about 15 million selected from copolymers acrylic acid, methacrylic acid, isocrotonic acid and combinations thereof. |
| US5554147 A | CApHCO, Inc. | Compositions and devices for controlled release of active ingredients | A method for the controlled release of a biologically active agent wherein the agent is released from a hydrophobic, pH-sensitive polymer matrix is disclosed and claimed. The polymer matrix swells when the environment reaches pH 8.5, releasing the active agent. A polymer of hydrophobic and weakly acidic comonomers is disclosed for use in the controlled release system. Further disclosed is a specific embodiment in which the controlled release system may be used. The pH-sensitive polymer is coated onto a latex catheter used in ureteral catheterization. A common problem with catheterized patients is the infection of the urinary tract with urease-producing bacteria. In addition to the irritation caused by the presence of the bacteria, urease produced by these bacteria degrade urea in the urine, forming carbon dioxide and ammonia. The ammonia causes an increase in the pH of the urine. Minerals in the urine begin to precipitate at this high pH, forming encrustations which complicate the functioning of the catheter. A ureteral catheter coated with a pH-sensitive polymer having an antibiotic or urease inhibitor trapped within its matrix will release the active agent when exposed to the high pH urine as the polymer gel swells. Such release can be made slow enough so that the drug remains at significant levels for a clinically useful period of time. |
| US20030153983 A1 | SCIMED LIFE SYSTEMS INC | Implantable or insertable medical device resistant to microbial growth and biofilm formation | Disclosed are implantable or insertable medical devices that provide resistance to microbial growth on and in the environment of the device and resistance to microbial adhesion and biofilm formation on the device. In particular, the invention discloses implantable or insertable medical devices that comprise at least one biocompatible matrix polymer region, an antimicrobial agent for providing resistance to microbial growth and a microbial adhesion/biofilm synthesis inhibitor for inhibiting the attachment of microbes and the synthesis and accumulation of biofilm on the surface of the medical device. Also disclosed are methods of manufacturing such devices under conditions that substantially prevent preferential partitioning of any of said bioactive agents to a surface of the biocompatible matrix polymer and substantially prevent chemical modification of said bioactive agents |
Urinary Problems in men and women
- Both men and women have an increased risk for urinary incontinence as they get older, with men's rates rising steadily and women's rates peaking during menopause.
- The prevalence of incontinence in men of all ages is certainly lower than that for women.
- Women over 70, however, are twice as likely to have urinary incontinence as men of the same age.
Source: Urinary prevalence men Vs women
Market Analysis
- We determined market data to have an idea about the market potential for ureteral stents.
- We have done this modeling for female population in US as women has the higher prevalence rate for urinary incontinence than men in all age groups.
- Prevalence increased with age, from 28% for 30- to 39-year-old women to 55% for 80- to 90-year-old women.
- 18% of respondents reported severe UI.
- The prevalence of severe UI also increased notably with age, from 8% for 30- to 39-year-old women to 33% for 80- to 90-year-old women.
- Among all, 9% reported slight UI, 15% reported moderate UI, 18% reported severe UI, and 58% reported no UI.
Methodology
Prevalence rate in US (women)
| Prevalence of Urinary Incontinence in US (women) | |
| Age (in yrs) | Population with Urinary incontinence (in %) |
| 30-39 | 28% |
| 40-49 | 41% |
| 50-59 | 48% |
| 60-69 | 51% |
| 70-79 | 55% |
| 80-90 | 54% |
Source: Archives of internal medicine
Urinary incontinence severity among different age groups in US women
Market potential for ureteral stent in US (women)
| Market potential for ureteral stents in US women, 2009 | |||||
| 1. Age groups |
2. Female population (from US census data) |
3. Prevalence rate in female (%) | 4. Market potential (total prevalence) (2*3) |
5. Catherization rate (%) |
6. Stent market based on catherization rate (4*5) |
| 30-39 | 20128402 | 28% | 5635953 | 0.043% | 2423 |
| 40-49 | 22074384 | 41% | 9050497 | 0.123% | 11132 |
| 50-59 | 20929761 | 48% | 10046285 | 0.124% | 12457 |
| 60-69 | 14605565 | 51% | 7448838 | 0.160% | 11918 |
| 70-79 | 9046207 | 55% | 4975414 | 0.172% | 8558 |
| ≥ 80 | 7216598 | 54% | 3896963 | 0.044% | 1715 |
| Total | 41053950 | 48203 | |||
- Prevalence rate in US women is growing at a CAGR of 1.19%
- Ureteral stent market is growing at a CAGR of 1.47%
Ureteral stent market forecast in US (women)
Ureteral stent companies
Various companies offering ureteral stents are:
Ureteral stents of various companies
Boston Scientific
- Contour VL Variable Length Percuflex Stents
Inward spiral design of Nautilus Coil minimizes tissue contact for enhanced comfort. Dual variable length coil geometry permits balanced stent positioning to minimize the risk of migration. HydroPlus Coating provides unequalled surface lubricity which reduces friction to minimize risk of buckling during introduction and placement and to reduce risk of trauma and encrustation.
- Percuflex Stents
- High coil strength pigtail shape prevent stent migration
- Thin wall promotes drainage and patency
- Multiple, large side ports promote drainage
- Attached suture for positioning and subsequent removal without the need for repeat cystoscopy
- Polaris Ultra Ureteral Stent
- Ultra Ureteral Stent provides the ease of placement benefits of a firm stent graduated into a soft bladder coil
- Co-extrusion combines a firm durometer Percuflex Plus Material and a soft durometer Percuflex Material in the same stent
- Nautilus bladder coil potentially reduce bladder irritation, and a relaxed renal coil facilitate ease of removal
Cook Group
- Bander Ureteral Diversion Stent Set
It is used for intraoperative placement to stent the ureter during ureteroileal conduit construction and continent urinary diversions. Set includes: 2 stents, 2 catheter retainers and wire guide.
- C-Flex Double Pigtail Ureteral Stent Set
It is used for temporary internal drainage from the ureteropelvic junction to the bladder. Set includes stent, wire guide, stent positioner and catheter.
- Towers Peripheral Ureteral Stent Set
It is also used for temporary internal drainage from the ureteropelvic junction to the bladder. The stent configuration allows peripheral as well as luminal drainage. Set Includes: Stent, Wire Guide, Catheter, and Stent Positioner.
Applied medicals
- 7-10 endopyelotomy stent
It is used by urologists for endopyelotomy and endoureterotomy. The dual diameter promotes optimal healing while minimizing the discomfort often associated with larger diameter stents.
Redi-Tech Medical Products
- Ureteral stents set
- Attached suture for positioning and subsequent removal without the need for repeat cystoscopy
- Multiple, large side ports promote drainage
- Radiopaque stent markings aid in placement and sizing
Like this report?
This is only a sample report with brief analysis
Dolcera can provide a comprehensive report customized to your needs
Clinical Trials
New trials
| Title | Conditions | Intervention | Sponsors and Collaborators |
| Assessment of Drug-Eluting Ureteral Stent on Bacterial Adherence and Biofilm Formation | Renal Calculi, Ureteral Obstruction | Device: Ureteral Stent | Lawson Health Research Institute, Boston Scientific Corporation |
| Memokath® 044TW Stent for Treatment of Urethral Stricture | Urethral Stricture | Device: Memokath stenting | Engineers & Doctors Wallsten Medical Group |
| Study to Determine if There Are Specific Clinical Factors to Determine Stent Encrustation | Kidney Stones | N\A | University of California, Irvine |
| Ureteral Stent Length and Patient Symptoms | Kidney Stones | Device: Ureteral Stent | Emory University |
| Drainage of Malignant Extrinsic Ureteral Obstruction Using the Memokath Ureteral Stent | Ureteral Obstruction | Device: Memokath 051 Ureteral Stent | Mayo Clinic Engineers & Doctors Wallsten Medical Group |
| A Prospective Comparison Between Ureteral Stent and Nephrostomy Tube for an Urgent Drainage of Obstructed Kidney (JJVsPCN08) | Kidney Disease | Device: nephrostomy tube and ureteral stent | Rabin Medical Center |
Concluded trials
| Title | Abstract | Enrollment | Disorder | Conclusion |
| Long-term outcome of permanent urethral stents in the treatment of detrusor-sphincter dyssynergia | To evaluate the long-term efficacy of a permanently implanted urethral stent in the treatment of spinally injured patients with detrusor-sphincter dyssynergia. | 13 | Detrusor-sphincter dyssynergia | Stenting is an effective alternative to sphincterotomy in the long-term, although secondary bladder neck obstruction is a frequent problem. |
| Nephrostomy Tube or 'JJ' Ureteric Stent in Ureteric Obstruction: Assessment of Patient Perspectives Using Quality-of-Life Survey and Utility Analysis | Upper urinary tract obstruction is often relieved by either a percutaneous nephrostomy tube (PCN) or a ureteric stent. Both can cause considerable morbidity and reduce patient's health-related quality of life (QoL). We have compared the QoL in these 2 groups. | 34 | Upper urinary tract obstruction | Patients with 'JJ' stents have significantly more irritative urinary symptoms and a high chance of local discomfort than patients with nephrostomy tubes (PCN). However, based on the EuroQol analysis, there is no significant difference in the gross impact on the health-related QoL or the utility between these groups indicating no patient preference for either modality of treatment. |
| Impact of stents on urological complications and health care expenditure in renal transplant recipients: results of a prospective, randomized clinical trial. | A randomized, prospective trial to compare the incidence of early urological complications and health care expenditures in renal transplant recipients with or without ureteral stenting. | 201 | Renal transplant recipient | Using a ureteral stent at renal transplantation significantly decreases the early urinary complications of urine leakage and obstruction. However, there is a significant increase in urinary tract infections, primarily beyond 30 days after transplantation. Stent removal within 4 weeks of insertion appears advisable. |
Adverse Events
| S. No. | Brand Name | Adverse Event | Date FDA Received |
| 1 | Cook Urologicals Cook Urological Stent | Stent broke into pieces while removing it from the patients body. | 12/14/2005 |
| 2 | Boston Scoientific Boston Scientific Ureteral stent System | Fractured stent seen under Fluroscopy | 10/17/2007 |
| 3 | Boston Scoientific Boston Scientific Ureteral Stent System Kit 8 FR X 24 CM | During insertion of ureteral stent, the stent broke into multiple parts which were retained in the patient. | 10/14/2005 |
| 4 | Boston Scientific Corp Boston Scientific 8 FR X 28 CM Ureteral Stent System Kit | Breakage of the upper loop of the ureteral stent while trying to insert it. | 1/5/2005 |
| 5 | Boston Scientific Bostoon Scientific Micro Vasive Contour VL Ureteral Stent | Broken stent observed during x-ray procedure. | 12/12/2003 |
Review Articles
Non Patent Analysis
Products
| Boston Scientific Scimed, Inc. | Cook Urological Incorporated | OptiMed Global Care |
| Polaris™ Ultra Ureteral Stent | Firlit-Kluge Urethral Stent | Opti-J Ureteral Stent System |
| Polaris™ Loop Ureteral Stent | Koyle Diaper Stent | Ureteral Stent Sets, ureterorenoscope |
| Stretch™ VL Variable Length Flexima® Stents | Silicone Universal Drainage Stent | Extra Strong Stent Sets (-Tumor) |
| Tarkington Urethral Stent Set | Steerable Ureteral Stent Sets | |
| Zaontz Urethral Stent | Multilength | |
| Pediatric Urethral C-Stent |
Startup activity
- AbbeyMoor Medical Inc., a med-tech firm that’s developed devices for treating urological disorders, has raised $2.7 million in bridge financing.
Phase 2: Deeper Dive
Scenario
Client wishes to acquire a ureteral stent company.
Deal analysis for a target company
Design History File Review: Review components
| Review | Verification | Tasks | Expertise |
|---|---|---|---|
| Design Input | Design input documents for sufficiency |
|
Quality systems |
| Design input documents linked to the product performance specifications |
| ||
| Product Performance Specifications (PPS) | Design inputs correlate adequately to the specifications; DV&V (design verification and validation) criteria are based on risk management documentation or if the criteria are based on sound statistical sampling plans |
|
Quality systems, CAD |
| Appropriate design verification and validations (DV&V) are performed |
| ||
| Product performance specifications correspond to appropriate design output documents |
| ||
| Risk Management Documents | Risk Analysis, Design Failure Modes and Effects Analysis (DFMEA), Process FMEA, other risk management documentation |
|
Quality systems |
| DFMEA links appropriately to the PPS |
| ||
| Appropriate DV&V reports and design output documents are referenced correctly as risk mitigation activities in the DFMEA |
| ||
| PFMEA links appropriately to the process validation protocol acceptance criteria; In-process inspection procedures and/or manufacturing procedures are recorded as appropriate risk mitigation activities in the PFMEA |
| ||
| Design Output Documents | Completeness of drawings |
|
Quality systems, CAD |
| Correlate First Article Inspection data to the dimensions on the drawings |
| ||
| Manufacturing Documents | Manufacturing procedures, component specifications, raw material specifications, incoming and in-process inspection procedures for completeness |
|
Material science, manufacturing engineering, quality systems |
| Linkage between component and raw material specifications and appropriate incoming inspection procedures |
| ||
| Inspection procedures have adequate sampling plans based on PFMEA risk mitigation levels – this includes packaging and labeling materials |
| ||
| Calibration records and preventive maintenance records; in-process / incoming inspection test methods and related test method validations |
| ||
| Validation Report | DV&V reports, Shelf-life reports, Biocompatibility test reports, Sterilization reports, Packaging Validation reports, Process Validation Reports |
|
Quality systems |
| Design test methods and related test method validations |
|
Sample report
Performance/Functional Characteristics
| Design Input | Design Output | Design Verification Report # | Status (P/F/R) | Design Validation Report # | Status (P/F/R) | |||
|---|---|---|---|---|---|---|---|---|
| User Needs | User Need Rationale | Engineering Specification | Engineering Specification Rationale | |||||
| Provide antimicrobial resistance for up to 2 weeks | Ureteral Stent User Survey (Document #XXXXX) | Stent must have chlorohexadine surface concentration of 10-20 mg/cm2 for 3 weeks | Document #XXXXX | Test Document #XXXXX | Report 01-005-06-007 | P | Report 01-005-06-007 | P |
Potential DHF Review Outcomes
Based on a review of the above DHF documents a potential outcome for the uretral stent acquisition project could involve the following:
- Better explanation of existing design input documents and also better linkage between the design inputs and product specifications.
- Creation of some new test methods for design, incoming and in-process inspections and also include recommendations for the test method validations. Creation of any new DV&V data would be highly unlikely as it could potentially trigger a new submission or a note-to-file to the regulatory agencies.
- Change in raw materials to better grade materials e.g. Switching resin to a USP Class VI biocompatible resin. This would eliminate some on-going testing but require additional upfront one time biocompatibility testing.
- Updating drawings based on results from the FAI data.
- Converting existing Company Y documents into Company X format and identifying potential gaps and streamlining linkage between raw material specifications and inspection procedures.
- Identifying installation, operational and process qualification requirements with the assumption that no additional design verification and validation activities are required based on the fact that the device is currently approved for sale in the US and ROW.
- Recommend activities necessary for completing packaging, labeling, ship testing and shelf-life testing. Stress should be on being able to leverage existing data for shelf-life without changing the regulatory status of the device.
- Company X may want to perform additional biocompatibility testing to create an internal baseline and also update their biocompatibility files.
- Help streamline suppliers for components when switching over from Company Y to Company X. Search for existing Company X suppliers that can supply off the shelf items that Company Y may be sourcing from other vendors / suppliers.
- Identify process improvements that can be rolled into the manufacturing transfer without changing the design and impacting the existing regulatory status for the device e.g. instead of hand mixing pigment to resin use a pre-mixer to control quality of mixing and resulting extrusion or perform the molding and over-molding steps in 1 machine instead of 2 separate molding machines.
Phase 3: Post-acquisition integration
Deadlines
Goal: Switch production transparently to new facilities transparently to the distribution system
| Stage | Tasks | Milestone payment | Date |
|---|---|---|---|
| Design center integration plan |
|
September 15, 2008 | |
| Design to manufacturing transfer | December 15, 2008 | ||
| Equipment transfer | Milestone I payment | Jan 7, 2009 | |
| Shut production at acquiree facility | Negotiation for contract extension | Milestone II payment | Feb 15, 2009 |
| Start production in acquirer facility | Feb 7, 2009 | ||
| Switch to new SKU | Feb 15, 2009 | ||
| End development of new generation product/s in old facility | Feb 7, 2009 | ||
| Restart development of new generation product/s post-acquisition | Final milestone payment | Mar 1, 2009 |
Documents and Ownership
| Document | Owner | Last update date |
|---|---|---|
| Product performance specifications | Paul Swain | 07/27/2008 08:15:35 PST |
| Component specifications | Kevin Teller | 06/12/2008 12:22:07 PST |
| Preclinical test results | Joanne Krannert | 07/03/2008 14:17:00 PST |
| Clinical tests | Joanne Krannert | 08/01/2008 08:00:55 PST |
Ureteral stents regulatory issues
The FDA classifies a ureteric stent as follows:
- TITLE 21 - FOOD AND DRUGS
- CHAPTER I - FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES
- SUBCHAPTER H - MEDICAL DEVICES
- PART 876 - GASTROENTEROLOGY-UROLOGY DEVICES
- Subpart E - Surgical Devices
- Sec. 876.4620 - Ureteral stent.
- Classification - class II device Code of Federal Regulations
Pre-Market Notification
Section 510(k) of the Food, Drug and Cosmetic Act requires device manufacturers who must register, to notify FDA of their intent to market a medical device at least 90 days in advance, also known as Premarket Notification. This premarket submission demonstrates to the FDA that the device to be marketed is atleast as safe and effective, that is, substantially equivalent, to a legally marketed device. Parties required to submit a 510(k) to the FDA include domestic or foreign manufacturers introducing a device to the U.S. market, as well as specification developers and repackers/relabelers.
A 510(k) is required when:
- Introducing a device into commercial distribution (marketing) for the first time.
- Proposed different intended use for a device already in commercial distribution.
- Change or modification of a legally marketed device.
510(k) “Substantial Equivalence” Decision Making Process
Some of the companies active in the field of ureteral stents have been represented in the table below. (This is not an exhaustive list and is just a sample)
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
Silicone | Double pigtail with monofilament suture loop | |
|
| |
|
|
|
|
Dual Durometer Percuflex with HydroPlus Coating | Bladder loop design | |
|
| |
|
|
|
|
Metal | Temporary stenting | |
|
| |
|
|
|
|
Polyurethane | Spiral radially expanding stent | |
|
| |
| |||||||
| |
|
|
|
|
Nickel-titanium shape memory alloy | Double fluted ended spiral stent | |
Timeline Sheet
- References
1- http://en.wikipedia.org/wiki/Demographics_of_the_United_States
2- http://www.wrongdiagnosis.com/c/catheter_infection/stats.htm?ktrack=kcplink
3- http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/WomensHealthResearch/UCM247851.pdf
Like this report?
This is only a sample report with brief analysis
Dolcera can provide a comprehensive report customized to your needs
Contact Dolcera
| Samir Raiyani |
|---|
| Email: info@dolcera.com |
| Phone: +1-650-269-7952 |
Addition
Patent Categorization: Interactive mind map linked to Dolcera Dashboard
- To access the Dashboard you have to signup. You can do so by clicking here
- Use the mouse(click and drag/scroll up or down/click on nodes) to explore nodes in the detailed taxonomy
- Click on the red arrow adjacent to the node name to view the content for that particular node in the dashboard
- Click on the "+" sign to zoom the mindmap and "-" sign to shrink the mindmap
Product-Patent-Clinical Trials Mapping
- To access the Dashboard you have to signup. You can do so by clicking here
- Use the mouse(click and drag/scroll up or down/click on nodes) to explore nodes in the detailed taxonomy
- Click on the red arrow adjacent to the node name to view the content for that particular node in the dashboard
- Click on the "+" sign to zoom the mindmap and "-" sign to shrink the mindmap
Product to Patent Mapping
- Click here to download the excel file.
- Mapped Patent vs Not Mapped Patents
Dolcera Dashboard
Dashboard Link
| Ureteral Stent - Dashboard |
- Flash Player is essential to view the Dolcera Dashboard
- To access the Dashboard you have to signup. You can do so by clicking here
Key Artifacts
- Investment Heat Map
- Revenue Heat Map
- Patent Heat Map
- Dynamic Patent Dashboard
- Stent Landscape-Flash
- Company Profile - Flash