Difference between revisions of "Ureteral Stent"
(→Encrustation) |
(→The need) |
||
| Line 101: | Line 101: | ||
weeks, actually is adsorbed onto the stent which makes longer term treatment possible with reduced risk of bacterial resistance. There has been research targeted at coating or impregnating urinary catheters with antimicrobials and products are on the market, however, there are no antimicrobial ureteral stents approved by the FDA. | weeks, actually is adsorbed onto the stent which makes longer term treatment possible with reduced risk of bacterial resistance. There has been research targeted at coating or impregnating urinary catheters with antimicrobials and products are on the market, however, there are no antimicrobial ureteral stents approved by the FDA. | ||
| − | === The need === | + | === The market need === |
It is clear that there is a need for a new material that will be able to resist encrustation and infection in the urinary tract. According to Merrill Lynch, ureteral stents represent an $80 MM | It is clear that there is a need for a new material that will be able to resist encrustation and infection in the urinary tract. According to Merrill Lynch, ureteral stents represent an $80 MM | ||
| − | US market. Boston Scientific is in the lead with ~50% of the market followed by Maxxim (Circon), Cook and Bard is a smaller player. There are a number of other small contenders | + | US market. Boston Scientific is in the lead with ~50% of the market followed by Maxxim (Circon), Cook and Bard is a smaller player. There are a number of other small contenders. |
| − | + | ||
| − | + | ||
| − | + | ||
| + | The use of ureteral stents is increasing; the indications for ureteral stenting have broadened from temporary or permanent relief or ureteric obstruction to include temporary urinary diversion following surgical procedures such as endopyelotomy and ureteroscopy and facilitation of stone clearance after ESWL (Tolley, 2000). | ||
| + | The use of ureteral stents for patients having ESWL for renal calculi is however controversial and seems to be related to the size of the stones and invasiveness of the procedure. According to survey results reported by Hollowell, et al. (2000), there is a significant difference in opinion concerning the use of stents with ESWL. | ||
| − | + | The number of ureteral stents used in patients with stones 2 cm or less treated with ESWL is significant in spite of the lack scientific evidence in support of this practice. Of 1,029 urologists returning surveys, for patients with renal pelvic stones 10, 15 or 20 rnm treated with ESWL, routine stent placement was preferred by 25.3%, 57.1 % and 87.1 %, respectively. Urologists recommend using ureteroscopy rather than ESWL for distal ureteral calculi 5-1 0 mm. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | in | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | 5 | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
Revision as of 16:28, 1 August 2008
Contents
Ureteral Stent: Concept
An antimicrobial ureteral stent, which inhibits encrustation and bacterial colonization while maintaining patient comfort.
- Ureteral stent: resists migration, resists fragmentation, is kink resistant and radiopaque.
- Bacterial colonization: antimicrobial activity for up to two weeks.
- Patient Comfort: stent has a low coefficient of fiiction (value) for ease of insertion and will soften on implant at body temperature to maintain patient comfort.
Background
Ureteral stents are used in urological surgery to maintain patency of the ureter to allow urine drainage from the renal pelvis to the bladder. These devices can be placed by a number of different endourological techniques. They are typically inserted through a cystoscope and may also be inserted intraoperatively. Indwelling ureteral stents help to reduce complications and morbidity subsequent to urological and surgical procedures. Frequently, ureteral stents are used to facilitate drainage in conjunction with Extracorporeal Shock Wave Lithotripsy (ESWL) and after endoscopic procedures. They are also used to internally support anastomoses and prevent urine leakage after surgery. Ureteral stenting may almost eliminate the urological complications of renal transplantation.
The advent of ESWL and the more recent barrage of endourological techniques have increased the indications for ureteral stents (Candela and Bellman 1997). Indications for use include:
- Treatment of ureteral or kidney stones
- Ureteral trauma or stricture
- Genitourinary reconstructive surgery
- Hydronephrosis during pregnancy
- Obstruction due to malignancy
- Retroperitoneal fibrosis
The need for ureteral stents range from a few days to several months. For patients with serious urological problems, ureteral stent maintenance may become a life-long necessity. Unfortunately, there are many problems associated with using ureteral stents.
Ureteric stenting difficulties
| Common | Rare |
|
|
Today, elastomeric materials, such as silicones, polyurethanes and hydrogel-coated polyolefins are used, with no clear winner, which can withstand the urinary environment.
- Although silicone has better long-term stability than other stent materials, its extreme flexibility makes it difficult to pass over guidewires and through narrow or tortuous ureters.
- Polyethylene is stiffer and easier to use for patients with strictures; however, it has been known to become brittle with time leading to breakage and is no longer commercially available. * Polyurethane has properties that fall in between polyethylene and silicone; however, stent fracture also has been an issue with polyurethanes.
Attempts have been made to develop polymers with a combination of the best of all properties. The key players are C-Flex (Concept Polymer Technologies), Silitek and Percuflex (Boston Scientific).
- C-Flex is proprietary silicone oil and mineral oil interpenetrated into a styrenelolefin block copolymer with the hope of reduced encrustation.
- Silitek (Medical Engineering Corporation) is another silicone-based copolymer.
- Percuflex is a proprietary olefinic block copolymer.
Metallic stents have been used recently to treat extrinsic ureteric obstructions. The effect of synthetic polymers on the urothelium of the urinary tract seems to be dependent on the bulk chemical composition of the polymer, the chemical composition of its surface, coatings on the device surface, smoothness of the surface and coefficient of friction.
Typically, most ureteral stents are made of relatively smooth catheters. Koleski et al., (2000) tested a longitudinally grooved ureteral stent made by Circon in the pig ureter. The results indicated that the grooved stent led to better drainage than a conventional stent. Their opinion is that the ureter wall has a better chance of collapsing over a smooth surface than a grooved surface, especially when debris is present. Stoller (2000) had the same experience with the SpiraStent(Urosurge Corp.). This helical stent was superior at passing stones than a conventional smooth stent.
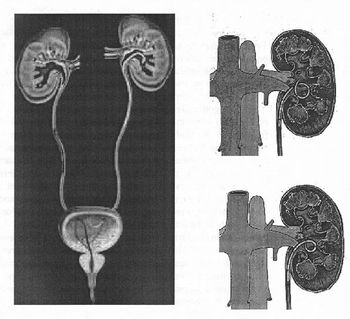
There are a variety of ureteral stent configurations with different anchoring systems. Most stents today have a double pigtail anchoring system. (Tolley, 2000), Dunn et al, (2000) conducted a randomized, single-blind study comparing a Tail stent (proximal pigtail with a shaft which tapers to a lumenless straight tail) to a double pigtail stent. The Tail stent was found to be better tolerated than the double-pigtail concerning lower urinary tract irritative symptoms. A double-J ureteral stent and a flexible ureteropyeloscope are shown in the first diagram. The other two diagrams show a pigtail ureteral stent in place; the end of the pigtail is facing away fiom the ureteral opening in the second of these two diagrams.
Early adverse effects of ureteral stenting include lower abdominal pain, dysuria, fever, urinary frequency, nocturia and hematuria. Patient discomfort and microscopic hematuria happen often. Major late complications include stent migration, stent fragmentation or more serious hydronephrosis with flank pain and infections.
Late complications occurred in one third of the patients in a prospective study using both silicone and polyurethane double pigtail stents (110 stents) in 90 patients. Stent removal was necessary in these patients. Others also have found this percentage of late complications. Device-related urinary tract infection and encrustation can lead to significant morbidity and even death and are the primary factors limiting long-term use of indwelling devices in the urinary tract. Microbial biofilm and encrustation may lead to stone formation. This is typically not a problem when stents are used for short-term indications. Problems of biofilm formation, encrustation and stent fracture occur in patients with long-term indwelling stents.
Typically, manufacturers advise periodic stent evaluation. Cook polyurethane stent removal is recommend at 6 months and 12 months for silicone (Cook product literature). However, stents that are intended for long-term use are usually changed at regular intervals, as frequently as every 3 months.
Forgotten stents are a problem. Monga et al., 1995 found that 68% of stents forgotten more than 6 months were calcified and 10% were fragmented. Multiple urologic procedures were necessary to remove the stones. Long-term effects of these forgotten stents may lead to voiding dysfunction and renal insufficiency. Schlick, et al., 1998 are developing a biodegradable stent that will preclude the need for stent removal.
Encrustation
The urinary system presents a challenge because of its chemically unstable environment. Long-term biocompatibility and biodurability of devices have been problems due to the supersaturation of uromucoids and crystalloids at the interface between urine and the device. Encrustation of ureteral stents is a well-known problem, which can be treated easily if recognized early. However, severe encrustation leads to renal failure and is difficult to manage (Mohan-Pillai et al., 1999). All biomaterials currently used become encrusted to some extent when exposed to urine.
The encrusted deposits can harbor bacterial biofilms. In addition, they can render the biomaterial brittle which causes fracture in-situ, a serious problem especially associated with the use of polyethylene and polyurethane ureteral stents (although silicone stents have also been reported to fracture). Stent fragments can migrate to the bladder or renal pelvis with serious repercussions.
Surface science techniques were used to study three stent types after use in patients. The stent type, duration of insertion and age or sex of the patient did not correlate significantly with the amount of encrustation (Wollin et al., 1998). However, it has been suggested that factors which affect the amount of encrustation include the composition or the urine, the type of invading and colonizing bacteria and the structure and surface properties of the biomaterial used (Gorman 1995). A low surface energy surface seems to resist encrustation compared with a high surface energy surface (Denstedt et al., 1998).
Many different types of stone can form in the urinary tract. Calcium oxalate, calcium phosphate, uric acid and cystine stones are metabolic stones because they form as a result of metabolic dysfunction. They usually are excreted from the urinary tract. Struvite (magnesium ammonium phosphate) and hydroxyapatite (calcium phosphate) are associated with infection (infection stones). These account for 1520% of urinary calculi. ESWL is used to break up the larger infection stones because they don't pass; recurrence of the problem occurs with incomplete removal. Infection stones can manifest as poorly mineralized matrix stones, highly mineralized staghorn calculi or as bladder stones which often form in the presence of ureteral stents. Urea-splitting bacteria colonize the surface and cause alkalinization of the urine, which lowers the solubility of struvite and hydroxyapatite, and they deposit on the surface. Bacterial biofilm associated with encrustation is a common clinical occurrence. (Gorman and Tunney, 1997). It has been suggested that prevention of bacterial colonization would prevent encrustation because of their ultimate responsibility for its formation (Bibby et al., 1995).
An in vitro model was developed that produces encrustation similar to those seen in vivo (Tunney et al., 1996a). An experiment was conducted to compare the encrustation potential of various ureteral stent materials. The long-term struvite and hydroxyapatite encrustation of silicone, polyurethane, hydrogel-coated polyurethane, Silitek and Percuflex were compared. All of the materials developed encrustation, however, it was found by image analysis that the rates of encrustation varied on the different materials. Silicone had less encrustation (69% at 10 weeks) compared to the other materials (1 00%) at the same time point (Tunney et al., 1996b). Continuous flow models have also been developed which are more representative of conditions in the upper urinary tract. They are discussed by Gorman and Tunney, (1 997). Efforts to reduce encrustation using new materials, smoother surfaces and hydrogel coatings have been attempted.
A hydrogel-coated C-flex stent (Hydroplus, Boston Scientific) was shown to have less epithelial cell damage and encrustation than other biomaterials and was recommended by the investigators for long-term use (Cormio, 1995). In addition, a poly(ethy1ene oxide)/polyurethane composite hydrogel (Aquavenem, J & J) resisted intraluminal blockage in a urine flow model compared with silicone and polyurethane (Gorman et al., 1997a). Another advantage with Aquavene is that it is rigid in the dry state, which facilitates insertion past obstructions in the ureter and becomes soft on hydration providing comfort (Gorman and Tunney, 1997). Gorman et al. (1997b) concluded that the chance of stent fracture would be reduced if the ureteral stent side holes were eliminated. Urinary tract infection is another common major problem with the usage of ureteral stents. Initially, a conditioning film is deposited on the ureteral stent surface. The film is made up of proteins, electrolyte materials and other unidentified materials that obscure the surface properties of the stent material. Electrostatic interactions, the ionic strength and pH of the urine and differences in fluid surface tensions affect bacterial adhesion to the conditioning film. Subsequently, a microbial biofilm forms over time. The biofilm is composed of bacterial cells embedded in a hydrated, predominantly anionic mixture of bacterial exopolysaccharides and trapped host extracellular macromolecules.
Obstruction
Obstruction of urine flow and urinary tract sepsis can result in continued growth of the biofilm. Colonization of devices implanted in the urinary tract can lead to dysfunction, tissue intolerance, pain, subclinical or overt infection and even urosepsis. Device related infections are difficult to treat and device removal is usually necessary. The biofilm has been found to impede the diffusion of antibiotics; in addition, the bacteria in the biofilm have a decreased metabolic rate , which also protects them against the effects of antibiotics (Wollin et al., 1998). Riedl, et al. (1 999) found 100% ureteral stent colonization rates in permanent and 69.3% in temporary stents. Antibiotic prophylaxis did not prevent bacterial colonization and it was recommended that it not be used. On the other hand, Tieszer, et al. (1 998) believe that fluoroquinolones can prevent infection. They also have found that some stents have denser encrustation than others, however, the stent material did not change the elements of the "conditioning film" adsorbed or alter its receptivity to bacterial biofilms.
Infection
The predictive value of urine cultures in the assessment of stent colonization was examined in 65 patients with indwelling ureteral stents. It was found that a sterile urine culture did not rule out the stent itself being colonized (Lifshitz, et al., 1999). Patients with sterile urine culture may benefit from prophylactic antibiotics; however, the authors contended that the antibiotics must work against gram-negative uropathogens and gram-positive bacteria including enterococci. It is obvious that there is controversy in the literature whether prophylactic systemic antibiotics are useful with ureteral stent implant. However, antibiotics do not seem to prevent stent colonization. Denstedt et al. (1998) have found that ciprofloxacin, with a 3 day burst every 2 weeks, actually is adsorbed onto the stent which makes longer term treatment possible with reduced risk of bacterial resistance. There has been research targeted at coating or impregnating urinary catheters with antimicrobials and products are on the market, however, there are no antimicrobial ureteral stents approved by the FDA.
The market need
It is clear that there is a need for a new material that will be able to resist encrustation and infection in the urinary tract. According to Merrill Lynch, ureteral stents represent an $80 MM US market. Boston Scientific is in the lead with ~50% of the market followed by Maxxim (Circon), Cook and Bard is a smaller player. There are a number of other small contenders.
The use of ureteral stents is increasing; the indications for ureteral stenting have broadened from temporary or permanent relief or ureteric obstruction to include temporary urinary diversion following surgical procedures such as endopyelotomy and ureteroscopy and facilitation of stone clearance after ESWL (Tolley, 2000).
The use of ureteral stents for patients having ESWL for renal calculi is however controversial and seems to be related to the size of the stones and invasiveness of the procedure. According to survey results reported by Hollowell, et al. (2000), there is a significant difference in opinion concerning the use of stents with ESWL.
The number of ureteral stents used in patients with stones 2 cm or less treated with ESWL is significant in spite of the lack scientific evidence in support of this practice. Of 1,029 urologists returning surveys, for patients with renal pelvic stones 10, 15 or 20 rnm treated with ESWL, routine stent placement was preferred by 25.3%, 57.1 % and 87.1 %, respectively. Urologists recommend using ureteroscopy rather than ESWL for distal ureteral calculi 5-1 0 mm.