Difference between pages "Wind Energy" and "Interferon For Treatment of Melanoma"
Crazypaddu (Talk | contribs) (→Patent Analysis) |
(→Search in Micropatent full text - English language search) |
||
| Line 1: | Line 1: | ||
| − | = | + | ==Dashboard== |
| − | + | ||
| − | + | ||
| − | * | + | * Click here to view '''[http://client.dolcera.com/dashboard/dashboard.html?workfile_id=890 Sample Dashboard]''' |
| − | + | NOTE: You need to install Internet Explorer 8.0 and Adobe Flash Player to view the Dashboard. | |
| − | + | Please download [http://www.microsoft.com/windows/internet-explorer/default.aspx '''Internet Explorer 8.0'''] and [http://get.adobe.com/flashplayer/?promoid=DRHWS '''Adobe Flash player'''] | |
| − | + | ==Objective== | |
| − | + | ||
| − | + | ||
| − | + | '''Primary objective of the study was to perform a prior art search on usage of interferon for the treatment of melanoma.''' | |
| − | + | To achieve our objective we performed following steps: | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | *Created a multi level taxonomy to categorize the patents using interferon for melanoma treatment | |
| − | + | ||
| − | + | ||
| − | * | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | *Marked out relevant IPC, ECLA, US classes and Japanese F-term available for technology in question. | |
| − | + | *Identified and clubbed relevant keywords with classes to extract relevant patents. | |
| − | + | *Checked for patents in US, EP, PCT, JP, Great Britain, and German patent records | |
| − | + | ||
| − | + | *Performed MPI-INPADOC search which cover bibliographic data for 71 countries and legal status for 42 countries | |
| − | + | *Analyzed the patents and prepared an IPmap covering relevant patents for client usage. | |
| − | == | + | ==Overview== |
| − | + | ||
| − | + | ===Interferon=== | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | [[Image:interferon.jpg|250px|right|thumb|'''Interferon''' [http://upload.wikimedia.org/wikipedia/commons/5/52/1HIG_Interferon-Gamma01.png Source]]] | |
| − | + | Interferons (IFNs) are natural cell-signaling proteins produced by the cells of the immune system of most vertebrates in response to challenges such as viruses, parasites and tumor cells. They belong to the large class of glycoproteins known as cytokines and are produced by a wide variety of cells in response to the presence of double-stranded RNA, a key indicator of viral infection. [http://en.wikipedia.org/wiki/Melanoma Source] | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | Interferons assist the immune response by inhibiting viral replication within host cells, activating natural killer cells and macrophages, increasing antigen presentation to T lymphocytes, and increasing the resistance of host cells to viral infection. There are 3 known classes of interferons; type I, type II and type III. All classes are very important in fighting viral infections. Recent studies have shown that Interferon can also help stop the growth and spread of cancer cells. [http://en.wikipedia.org/wiki/Interferon Source] | |
| − | + | ||
| − | + | ===Melanoma=== | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | Melanoma is the most serious type of skin cancer. It begins in skin cells called melanocytes. Melanocytes are the cells that make melanin, which gives skin its color. Melanin also protects the deeper layers of the skin from the sun's harmful ultraviolet (UV) rays.When people spend time in the sunlight, the melanocytes make more melanin and cause the skin to tan. This also happens when skin is exposed to other forms of ultraviolet light (such as in a tanning booth). If the skin receives too much ultraviolet light, the melanocytes may begin to grow abnormally and become cancerous. This condition is called melanoma.People with melanoma who have one or more positive lymph nodes are at a high risk to have their melanoma recur. It is believed that 70 to 80% of these individuals will have their melanoma come back within the next three to five years. [http://www.melanoma.com/whatis.html Source] | |
| − | + | ===Interferon for treatment of melanoma=== | |
| − | + | Over the past several decades, the incidence of melanoma has increased at a faster rate than that of any other solid tumor. In the 1930s, the lifetime risk for a person living in the U.S. to develop melanoma was 1 in 1,500. Currently, that risk is 1 in 74, and for 2003 it was estimated that 51,400 cases of invasive melanoma would be diagnosed. While efforts to improve early diagnosis through education have resulted in the increased detection of early-stage melanoma, many patients still present with high-risk primary melanomas. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | A beacon of hope in the treatment of melanoma has long been the observation that melanoma is susceptible to attack by the host’s immune system. This has resulted in the testing of a remarkably broad spectrum of immunotherapies, including the use of nonspecific immunostimulants, various approaches to vaccine therapies, and cytokine therapy. Many of these approaches failed to demonstrate a significant clinical impact, and the practitioner had been left with few options in treating high-risk melanoma patients with adjuvant therapy. One exception to this, however, has been the use of adjuvant interferon alpha (IFN-{alpha}) | |
| + | While the precise mechanism of action remains poorly understood, there are multiple antitumor effects of IFN-{alpha}. These include a direct antiproliferative effect, the enhancement of natural killer cell activity, and the upregulation of tumor antigens and/or HLA class I and class II antigens. Initial phase II clinical studies with IFN-{alpha} in metastatic melanoma showed response rates in the 10%–20% range [4, 5]. These response rates, while encouraging, were not significant enough to lead to its widespread use in the treatment of metastatic melanoma. [http://theoncologist.alphamedpress.org/cgi/content/full/8/5/451 Source] | ||
| − | |||
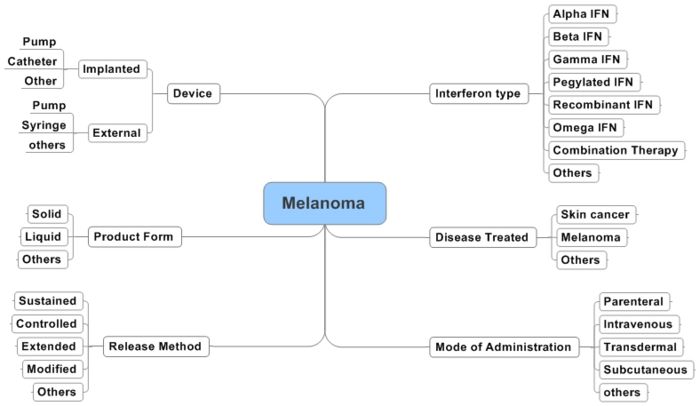
| − | [[ | + | ==Interactive Taxonomy== |
| + | <mm>[[Interferon_For_the_treatment_Of_Melanoma.mm]]</mm> | ||
| − | + | ==Concept Table== | |
| − | + | ||
| − | === | + | {|border="2" cellspacing="0" cellpadding="4" width="50%" |
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''S.No'''</center> | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''Concept-1'''</center> | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''Concept-2'''</center> | ||
| − | + | |- | |
| − | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''1'''</center> | |
| − | + | | style=";padding:0.079cm;"| <center>Melanoma</center> | |
| − | + | | style=";padding:0.079cm;"| <center>Interferon</center> | |
| − | |||
| − | |||
| − | |||
|- | |- | ||
| − | |''' | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''2'''</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Cancer</center> |
| + | | style=";padding:0.079cm;"| <center>IFN</center> | ||
| + | |||
|- | |- | ||
| − | | | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''3'''</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Skin Cancer</center> |
| + | | style=";padding:0.079cm;"| <center>huIFN</center> | ||
| + | |||
|- | |- | ||
| − | | ''' | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''4'''</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Carcinoma</center> |
| + | | style=";padding:0.079cm;"| | ||
| + | |||
|- | |- | ||
| − | | ''' | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''5'''</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Tumor</center> |
| + | | style=";padding:0.079cm;"| | ||
| + | |||
|- | |- | ||
| − | | ''' | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''6'''</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Melanocyte</center> |
| + | | style=";padding:0.079cm;"| | ||
|} | |} | ||
| − | + | ===French Keywords Concept table=== | |
| + | {|border="2" cellspacing="0" cellpadding="4" width="50%" | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''S.No'''</center> | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''Concept-1'''</center> | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''Concept-2'''</center> | ||
| − | = | + | |- |
| − | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''1'''</center> | |
| + | | style=";padding:0.079cm;"| <center>mélanome</center> | ||
| + | | style=";padding:0.079cm;"| <center>Interféron*</center> | ||
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
| − | | | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''2'''</center> |
| − | + | | style=";padding:0.079cm;"| <center>Peau Cancer </center> | |
| + | | style=";padding:0.079cm;"| <center>huIFN </center> | ||
| − | | | + | |- |
| − | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''3'''</center> | |
| − | | | + | | style=";padding:0.079cm;"| <center>Carcinome </center> |
| − | + | | style=";padding:0.079cm;"| <center>IFN </center> | |
| − | | | + | |
| − | + | ||
| − | === | + | |- |
| − | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''4'''</center> | |
| + | | style=";padding:0.079cm;"| <center>Tumeur </center> | ||
| + | | style=";padding:0.079cm;"| | ||
| − | + | |- | |
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''5'''</center> | ||
| + | | style=";padding:0.079cm;"| <center>Mélanocyte </center> | ||
| + | | style=";padding:0.079cm;"| | ||
| − | + | |} | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ===German Keywords Concept Table=== | |
| + | {|border="2" cellspacing="0" cellpadding="4" width="50%" | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''S.No'''</center> | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''Concept-1'''</center> | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''Concept-2'''</center> | ||
| − | ''' | + | |- |
| − | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''1'''</center> | |
| + | | style=";padding:0.079cm;"| <center>Melanoma</center> | ||
| + | | style=";padding:0.079cm;"| <center>Interferon</center> | ||
| − | ''' | + | |- |
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''2'''</center> | ||
| + | | style=";padding:0.079cm;"| <center>Haut Krebs </center> | ||
| + | | style=";padding:0.079cm;"| <center>huIFN </center> | ||
| − | + | |- | |
| − | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''3'''</center> | |
| − | + | | style=";padding:0.079cm;"| <center>Karzinoma </center> | |
| − | + | | style=";padding:0.079cm;"| <center>IFN </center> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | ''' | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | = | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | | | + | |
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | | | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''4'''</center> |
| − | + | | style=";padding:0.079cm;"| <center>Krebsgeschwür </center> | |
| − | | | + | | style=";padding:0.079cm;"| |
| − | | | + | |
|- | |- | ||
| − | | | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''5'''</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Tumor </center> |
| − | | | + | | style=";padding:0.079cm;"| |
| − | | | + | |
|- | |- | ||
| − | | | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>6</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Geschwulst </center> |
| − | + | | style=";padding:0.079cm;"| | |
| − | | < | + | |
| − | + | ||
| − | + | ||
| − | | | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|- | |- | ||
| − | | | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>7</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Melanozyten </center> |
| − | + | | style=";padding:0.079cm;"| | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | | | + | |
| − | + | ||
| − | + | ||
| − | | | + | |
| − | + | ||
| − | + | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | ===Class codes identified for searches=== | ||
| + | * '''Relevant IPC classes''' | ||
| − | + | {|border="2" cellspacing="0" cellpadding="4" width="75%" | |
| − | {|border="2" cellspacing="0" cellpadding="4" width=" | + | |align = "center" bgcolor = "#99CCFF" colspan = "4"|'''IPC''' |
| − | |align = "center" bgcolor = "# | + | |- |
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#C0C0C0"|'''Sr. No.''' |
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#C0C0C0"|'''Class Code''' |
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#C0C0C0"|'''Class definition''' |
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#C0C0C0"|'''Class coverage''' |
|- | |- | ||
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#C0C0C0"|'''1''' |
| − | | | + | |align = "center"|A61K003819 |
| − | + | |align = "center"|Medicinal preparations containing peptides - Cytokines; Lymphokines; Interferons | |
| − | + | |align = "center"|Broad | |
| − | | | + | |- |
| − | + | |align = "center" bgcolor = "#C0C0C0"|'''2''' | |
| − | + | |align = "center"|A61K003821 | |
| − | | | + | |align = "center"|Medicinal preparations containing peptides Interferon |
| − | + | |align = "center"|Specific | |
| − | | | + | |- |
| − | + | |align = "center" bgcolor = "#C0C0C0"|'''3''' | |
| + | |align = "center"|C07K001452 | ||
| + | |align = "center"|Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof - Cytokines; Lymphokines; Interferons | ||
| + | |align = "center"|Broad | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#C0C0C0"|'''4''' | ||
| + | |align = "center"|C07K014555 | ||
| + | |align = "center"|Peptides having more than 20 amino acids - Interferon | ||
| + | |align = "center"|Specific | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#C0C0C0"|'''5''' | ||
| + | |align = "center"|C07K001456 | ||
| + | |align = "center"|Peptides having more than 20 amino acids - IFN-alpha | ||
| + | |align = "center"|Specific | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#C0C0C0"|'''6''' | ||
| + | |align = "center"|C07K014565 | ||
| + | |align = "center"|Peptides having more than 20 amino acids - IFN-beta | ||
| + | |align = "center"|Specific | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#C0C0C0"|'''7''' | ||
| + | |align = "center"|C07K001457 | ||
| + | |align = "center"|Peptides having more than 20 amino acids - IFN-gamma | ||
| + | |align = "center"|Specific | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#C0C0C0"|'''8''' | ||
| + | |align = "center"|A61P003500 | ||
| + | |align = "center"|Therapeutic activity of chemical compounds or medicinal preparations -antineoplastic agents | ||
| + | |align = "center"|Broad | ||
|- | |- | ||
|} | |} | ||
| − | + | * '''Relevant ECLA classes''' | |
| − | + | ||
| − | + | {|border="2" cellspacing="0" cellpadding="4" width="75%" | |
| − | + | |align = "center" bgcolor = "#99CCFF" colspan = "4"|'''ECLA''' | |
| − | {|border="2" cellspacing="0" cellpadding="4" width=" | + | |
| − | | align = "center" bgcolor = "# | + | |
| − | + | ||
| − | + | ||
|- | |- | ||
| − | | align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#C0C0C0"|'''Sr. No.''' |
| − | | align = "center"| | + | |align = "center" bgcolor = "#C0C0C0"|'''Class Code''' |
| − | | | + | |align = "center" bgcolor = "#C0C0C0"|'''Class definition''' |
| + | |align = "center" bgcolor = "#C0C0C0"|'''Class coverage''' | ||
|- | |- | ||
| − | | align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#C0C0C0"|'''1''' |
| − | | align = "center"| | + | |align = "center"|A61K003819 |
| − | | | + | |align = "center"|Medicinal preparations containing peptides - Cytokines; Lymphokines; Interferons |
| + | |align = "center"|Broad | ||
|- | |- | ||
| − | | align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#C0C0C0"|'''2''' |
| − | | align = "center"| | + | |align = "center"|A61K003821 |
| − | | | + | |align = "center"|Medicinal preparations containing Interferon |
| + | |align = "center"|Specific | ||
|- | |- | ||
| − | | align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#C0C0C0"|'''3''' |
| − | | align = "center"| | + | |align = "center"|A61K38/21A |
| − | | | + | |align = "center"|Medicinal preparations containing IFN-alpha |
| + | |align = "center"|Specific | ||
|- | |- | ||
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#C0C0C0"|'''4''' |
| − | |align = "center"| | + | |align = "center"|A61K38/21B |
| − | | | + | |align = "center"|Medicinal preparations containing IFN-beta |
| + | |align = "center"|Specific | ||
|- | |- | ||
| − | | align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#C0C0C0"|'''5''' |
| − | | align = "center"| | + | |align = "center"|A61K38/21C |
| − | | | + | |align = "center"|Medicinal preparations containing IFN-gamma |
| + | |align = "center"|Specific | ||
|- | |- | ||
| − | | align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#C0C0C0"|'''6''' |
| − | | align = "center"| | + | |align = "center"|C07K001452 |
| − | | | + | |align = "center"|Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof - Cytokines; Lymphokines; Interferons |
| + | |align = "center"|Broad | ||
|- | |- | ||
| − | | align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#C0C0C0"|'''7''' |
| − | | align = "center"| | + | |align = "center"|C07K014555 |
| − | | | + | |align = "center"|Peptides having more than 20 amino acids - Interferon |
| + | |align = "center"|Specific | ||
|- | |- | ||
| − | | align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#C0C0C0"|'''8''' |
| − | | align = "center"| | + | |align = "center"|C07K001456 |
| − | | | + | |align = "center"|Peptides having more than 20 amino acids - IFN-alpha |
| + | |align = "center"|Specific | ||
|- | |- | ||
| − | | align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#C0C0C0"|'''9''' |
| − | | align = "center"| | + | |align = "center"|C07K014565 |
| − | | | + | |align = "center"|Peptides having more than 20 amino acids - IFN-beta |
| + | |align = "center"|Specific | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#C0C0C0"|'''10''' | ||
| + | |align = "center"|C07K001457 | ||
| + | |align = "center"|Peptides having more than 20 amino acids - IFN-gamma | ||
| + | |align = "center"|Specific | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#C0C0C0"|'''11''' | ||
| + | |align = "center"|C07K014715G | ||
| + | |align = "center"|Receptors; Cell surface antigens; Cell surface determinants - for interferons - | ||
| + | |align = "center"|Specific | ||
|- | |- | ||
| − | |||
| − | |||
| − | |||
|} | |} | ||
| − | + | * '''Relevant US classes''' | |
| − | + | ||
| − | = | + | {|border="2" cellspacing="0" cellpadding="4" width="50%" |
| − | + | |align = "center" bgcolor = "#99CCFF" colspan = "3"|'''US class''' | |
| − | ''' | + | |- |
| − | + | |align = "center" bgcolor = "#C0C0C0"|'''Sr. No.''' | |
| − | | | + | |align = "center" bgcolor = "#C0C0C0"|'''Class Code''' |
| + | |align = "center" bgcolor = "#C0C0C0"|'''Class definition''' | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#C0C0C0"|'''1''' | ||
| + | |align = "center"|4240854 | ||
| + | |align = "center"|DRUG, BIO-AFFECTING AND BODY TREATING COMPOSITIONS - this subclass provides for patents which broadly claim interferon or a method of treatment of interferon where the classification of the interferon as alpha, beta or gamma interferon is impossible | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#C0C0C0"|'''2''' | ||
| + | |align = "center"|4242811 | ||
| + | |align = "center"| DRUG, BIO-AFFCTING AND BODY TREATING COMPOSITIONS - Virus (e.g., interferon-inducing virus, etc.) | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#C0C0C0"|'''3''' | ||
| + | |align = "center"|42400141 | ||
| + | |align = "center"|DRUG, BIO-AFFECTING AND BODY TREATING COMPOSITIONS - Attached to lymphokine, cytokine, or other secreted growth regulatory factor, differentiation factor, or intercellular mediator specific for a hematopoietic cell (e.g., interferon, interleukin, macrophage factor, colony stimulating factor, erythropoietin); derivative thereof | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#C0C0C0"|'''4''' | ||
| + | |align = "center"|514889 | ||
| + | |align = "center"|DRUG, BIO-AFFECTING AND BODY TREATING COMPOSITIONS - INTERFERON INDUCER | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#C0C0C0"|'''5''' | ||
| + | |align = "center"|530351 | ||
| + | |align = "center"| CHEMISTRY: NATURAL RESINS OR DERIVATIVES; PEPTIDES OR PROTEINS; LIGNINS OR REACTION PRODUCTS THEREOF - Lymphokines, e.g., interferons, interlukins, etc. | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#C0C0C0"|'''6''' | ||
| + | |align = "center"|930142 | ||
| + | |align = "center"|PEPTIDE OR PROTEIN SEQUENCE - Interferon | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#C0C0C0"|'''7''' | ||
| + | |align = "center"|4240851 | ||
| + | |align = "center"|LYMPHOKINE - Included in this and the indented subclasses interferon, interleukin and macrophage factors (monokines) | ||
|- | |- | ||
| − | | align = "center"| | + | |align = "center" bgcolor = "#C0C0C0"|'''8''' |
| − | |align = "center"| | + | |align = "center"|4240855 |
| − | |align = "center"| | + | |align = "center"|Gamma or immune: This subclass is indented under subclass 85.4. Subject matter in which the interferon is gamma or immune interferon. |
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#C0C0C0"|'''9''' |
| − | |align = "center"| | + | |align = "center"|4240856 |
| − | |align = "center"| | + | |align = "center"|Subject matter in which the interferon is beta or fibroblast interferon. |
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#C0C0C0"|'''10''' |
| − | |align = "center"| | + | |align = "center"|4240857 |
| − | |align = "center"| | + | |align = "center"|Subject matter in which the interferon is alpha or leukocyte interferon. |
|- | |- | ||
|} | |} | ||
| − | |||
| − | = | + | ==Intellectual property== |
| − | + | ||
| − | + | ||
| − | + | ===Search strategy and concept=== | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | Date of Search: 1836 to Feb 3rd, 2011 | |
| − | + | Database used: Micropatent - Include extensive full text and MPI-Inpadoc searches | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ===Search in Micropatent full text - English language search=== | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | Micro patent full text search allow search in fulltext of US, EP, PCT, Great Britain, and German patent records as well as the front page of JP documents. US, EP, and DE are covered at first publication and when granted. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''S. No.''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Search concept''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Search Scope''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Search reason''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Class Code (IPC,US,ECLA)''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Search query''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''No. of hits''' | ||
|- | |- | ||
| − | | | + | |align = "center" bgcolor = "#99CCFF"|'''1''' |
| − | | | + | |align = "center"|Interferon for treating Melanoma |
| − | | | + | |align = "center"|Title, Abstract and Claims |
| − | | | + | |align = "center"|Specific classes of interferon AND melanoma keywords |
| − | | | + | |align = "center"|A61K003821<nowiki>*</nowiki> OR C07K014555 OR C07K001456 OR C07K014565 OR C07K001457 OR C07K014715G OR 4240854 OR 4242811 OR 42400141 OR 514889 OR 530351 OR 930142 |
| − | + | |align = "center"|(Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte<nowiki>*</nowiki> NEAR3 (cancer OR carcinoma OR tumor))) | |
| + | |align = "center"|576 | ||
|- | |- | ||
| − | | | + | |align = "center" bgcolor = "#99CCFF"|'''2''' |
| − | | | + | |align = "center"|Interferon for treating Melanoma |
| − | | | + | |align = "center"|Title, Abstract and Claims |
| − | | | + | |align = "center"|Broad classes of interferon AND melanoma, interferon keywords |
| − | | | + | |align = "center"|A61K003819 OR C07K001452 OR 4240851 OR 4240855 OR 4240856 OR 4240857 OR A61P003500 |
| − | + | |align = "center"|(Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte<nowiki>*</nowiki> NEAR3 (cancer OR carcinoma OR tumor))) | |
| + | |align = "center"|756 | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''3''' | ||
| + | |align = "center"|'''Final query''' | ||
| + | |align = "center" colspan = "4"|'''1 OR 2''' | ||
| + | |align = "center"|1019 records<br>'''571 unique records''' | ||
|- | |- | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|} | |} | ||
| − | == | + | ===Search in Micropatent full text - Foreign language search=== |
| − | + | ||
| − | + | ||
| − | |||
| − | {|border="2" cellspacing="0" cellpadding="4" width="100%" | + | Micro patent full text search allow search in fulltext of US, EP, PCT, Great Britain, and German patent records as well as the front page of JP documents. US, EP, and DE are covered at first publication and when granted. |
| − | |align = "center" bgcolor = "# | + | |
| − | |align = "center" bgcolor = "# | + | {|border="2" cellspacing="0" cellpadding="4" align = "center" width="100%" |
| + | |align = "center" bgcolor = "#99CCFF"|'''Sr. No.''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Search concept''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Language''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Search Scope''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Search reason''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Class Code (IPC, ECLA)''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Search query''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''No. of hits''' | ||
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#99CCFF" rowspan = "2"|'''1''' |
| − | | | + | |align = "center" rowspan = "2"|Interferon for treating Melanoma |
| + | |align = "center"|'''French''' | ||
| + | |align = "center" rowspan = "2"|Title, Abstract and Claims | ||
| + | |align = "center" rowspan = "2"|Specific classes of interferon AND melanoma<nowiki>’</nowiki>s foregin langugae keywords | ||
| + | |align = "center" rowspan = "2"|A61K003821<nowiki>*</nowiki> OR C07K014555 OR C07K001456 OR C07K014565 OR C07K001457 OR C07K014715G | ||
| + | |align = "center"|(mélanome or (Peau NEAR3 (Cancer or Carcinome or Tumeur)) OR (Mélanocytes Near3 (Cancer or Carcinome or Tumeur))) | ||
| + | |align = "center" rowspan = "2"|184 hits | ||
|- | |- | ||
| − | |align = "center"| | + | |align = "center"|'''German''' |
| − | | | + | |align = "center"|(Melanom or (Haut NEAR3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst)) OR (Melanozyten Near3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst))) |
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#99CCFF" rowspan = "2"|'''2''' |
| − | | | + | |align = "center" rowspan = "2"|Interferon for treating Melanoma |
| + | |align = "center"|'''French''' | ||
| + | |align = "center" rowspan = "2"|Title, Abstract and Claims | ||
| + | |align = "center" rowspan = "2"|Broad classes of interferon AND melanoma<nowiki>’</nowiki>s and interferon<nowiki>’</nowiki>s foregin langugae keywords | ||
| + | |align = "center" rowspan = "2"|A61K003819 OR C07K001452 OR A61P003500 | ||
| + | |align = "center"|(mélanome or (Peau NEAR3 (Cancer or Carcinome or Tumeur)) OR (Mélanocytes Near3 (Cancer or Carcinome or Tumeur))) | ||
| + | |align = "center" rowspan = "2"|3375 hits | ||
|- | |- | ||
| − | |align = "center"| | + | |align = "center"|'''German''' |
| − | | | + | |align = "center"|(Melanom or (Haut NEAR3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst)) OR (Melanozyten Near3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst))) |
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#99CCFF"|'''3''' |
| − | | | + | |align = "center" colspan = "5"|'''Final query''' |
| + | |align = "center"|'''1 OR 2''' | ||
| + | |align = "center"|'''3422 hits<br>(2023 unique records, 30-35 % relevant)''' | ||
|- | |- | ||
|} | |} | ||
| − | === | + | |
| + | ===Search in Micropatent MPI-INPADOC - English language search=== | ||
| + | |||
| + | Micrpatent MPI-INPADOC search bibliographic data for 71 countries and legal status for 42. Only those patents were analyzed which have English title and/or abstract. | ||
{|border="2" cellspacing="0" cellpadding="4" width="100%" | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#99CCFF"|'''Sr. No.''' |
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#99CCFF"|'''Search concept''' |
| + | |align = "center" bgcolor = "#99CCFF"|'''Search Scope''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Search reason''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Class search''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Search query''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''No. of hits''' | ||
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#C0C0C0"|'''1''' |
| − | | | + | |align = "center"|Interferon for treating Melanoma |
| + | |align = "center"|Title and Abstract | ||
| + | |align = "center"|Specific IPC classes of interferon AND melanoma keywords | ||
| + | |align = "center"|A61K03821 OR C07K014555 OR C07K01456 OR C07K014565 OR C07K01457 | ||
| + | |align = "center"|(Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte<nowiki>*</nowiki> NEAR3 (cancer OR carcinoma OR tumor))) | ||
| + | |align = "center"|174 | ||
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#C0C0C0"|'''2''' |
| − | | | + | |align = "center"|Interferon for treating Melanoma |
| + | |align = "center"|Title and Abstract | ||
| + | |align = "center"|Broad IPC classes of interferon AND melanoma, interferon keywords | ||
| + | |align = "center"|A61K03819 OR C07K01452 OR A61P03500 | ||
| + | |align = "center"|(IFN<nowiki>*</nowiki> OR <nowiki>*</nowiki>IFN OR interferon<nowiki>*</nowiki> OR <nowiki>*</nowiki>interferon OR huIFN) AND (Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte<nowiki>*</nowiki> NEAR3 (cancer OR carcinoma OR tumor))) | ||
| + | |align = "center"|484 | ||
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#C0C0C0"|'''3''' |
| − | | | + | |align = "center"|Interferon for treating Melanoma |
| + | |align = "center"|Title and Abstract | ||
| + | |align = "center"|Specific ECLA classes of interferon AND melanoma keywords | ||
| + | |align = "center"|A61K03821<nowiki>*</nowiki> OR C07K014555 OR C07K01456 OR C07K014565 OR C07K01457 OR C07K014715G | ||
| + | |align = "center"|(Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte<nowiki>*</nowiki> NEAR3 (cancer OR carcinoma OR tumor))) | ||
| + | |align = "center"|102 | ||
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#C0C0C0"|'''4''' |
| − | | | + | |align = "center"|Interferon for treating Melanoma |
| + | |align = "center"|Title and Abstract | ||
| + | |align = "center"|Broad ECLA classes of interferon AND melanoma, interferon keywords | ||
| + | |align = "center"|A61K03819 OR C07K01452 OR A61P03500 | ||
| + | |align = "center"|(IFN<nowiki>*</nowiki> OR <nowiki>*</nowiki>IFN OR interferon<nowiki>*</nowiki> OR <nowiki>*</nowiki>interferon OR huIFN) AND (Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte<nowiki>*</nowiki> NEAR3 (cancer OR carcinoma OR tumor))) | ||
| + | |align = "center"|9 | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#C0C0C0"|'''5''' | ||
| + | |align = "center" colspan = "4"|'''Final query''' | ||
| + | |align = "center"|'''1 OR 2 OR 3 OR 4''' | ||
| + | |align = "center"|587 hits<br>'''232 unique records''' | ||
|- | |- | ||
|} | |} | ||
| + | ===Search in Micropatent MPI-INPADOC - Foreign language search=== | ||
| + | Micrpatent MPI-INPADOC search bibliographic data for 71 countries and legal status for 42. Only those patents were analyzed which have English title and/or abstract. | ||
| − | + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | |
| − | {|border="2" cellspacing="0" cellpadding="4" width=" | + | |align = "center" bgcolor = "#99CCFF"|'''Sr. No.''' |
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#99CCFF"|'''Search concept''' |
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#99CCFF"|'''Language''' |
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#99CCFF"|'''Search Scope''' |
| − | |align = "center" bgcolor = "# | + | |align = "center" bgcolor = "#99CCFF"|'''Search reason''' |
| + | |align = "center" bgcolor = "#99CCFF"|'''Class Code (IPC, ECLA)''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Search query''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''No. of hits''' | ||
|- | |- | ||
| − | |align = "center"|1 | + | |align = "center" bgcolor = "#99CCFF" rowspan = "2"|'''1''' |
| − | | | + | |align = "center" rowspan = "2"|Interferon for treating Melanoma |
| − | | | + | |align = "center"|'''French''' |
| − | | | + | |align = "center" rowspan = "2"|Title and Abstract |
| + | |align = "center" rowspan = "2"|Specific IPC/ECLA classes of interferon AND melanoma keywords | ||
| + | |align = "center" rowspan = "2"|A61K03821 OR C07K014555 OR C07K01456 OR C07K014565 OR C07K01457 | ||
| + | |align = "center"|(mélanome or (Peau NEAR3 (Cancer or Carcinome or Tumeur)) OR (Mélanocytes Near3 (Cancer or Carcinome or Tumeur))) | ||
| + | |align = "center" rowspan = "2"|4 hits | ||
|- | |- | ||
| − | |align = "center"| | + | |align = "center"|'''German''' |
| − | | | + | |align = "center"|(Melanom or (Haut NEAR3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst)) OR (Melanozyten Near3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst))) |
| − | + | ||
| − | | | + | |
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#99CCFF" rowspan = "2"|'''2''' |
| − | | | + | |align = "center" rowspan = "2"|Interferon for treating Melanoma |
| − | | | + | |align = "center"|'''French''' |
| − | | | + | |align = "center" rowspan = "2"|Title and Abstract |
| + | |align = "center" rowspan = "2"|Broad IPC classes of interferon AND melanoma, interferon keywords | ||
| + | |align = "center" rowspan = "2"|A61K03819 OR C07K01452 OR A61P03500 | ||
| + | |align = "center"|(IFN<nowiki>*</nowiki> OR <nowiki>*</nowiki>IFN OR Interféron<nowiki>*</nowiki> OR <nowiki>*</nowiki>Interféron OR huIFN) AND (mélanome or (Peau NEAR3 (Cancer or Carcinome or Tumeur)) OR (Mélanocytes Near3 (Cancer or Carcinome or Tumeur))) | ||
| + | |align = "center" rowspan = "2"|25 hits | ||
|- | |- | ||
| − | |align = "center"| | + | |align = "center"|'''German''' |
| − | | | + | |align = "center"|(IFN<nowiki>*</nowiki> OR <nowiki>*</nowiki>IFN OR interferon<nowiki>*</nowiki> OR <nowiki>*</nowiki>interferon OR huIFN AND (Melanom or (Haut NEAR3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst)) OR (Melanozyten Near3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst))) |
| − | + | ||
| − | | | + | |
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#99CCFF"|'''3''' |
| − | | | + | |align = "center" colspan = "5"|'''Final query''' |
| − | | | + | |align = "center"|'''1 OR 2''' |
| − | | | + | |align = "center"|'''29 hits''' |
|- | |- | ||
|} | |} | ||
| − | ===Search | + | ===Search in Japanese database=== |
| − | + | Database: IPDL (Industrial property digital library), Japan | |
| + | Date of search: 1900/01/01 to 2011/02/15 | ||
| + | |||
| + | {| border="2" cellspacing="0" cellpadding="4" align = "left" width="50%" | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| '''S.No.''' | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Issue/Publication date'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''F-Term Theme'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''FI/F-term/Facet '''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Hits'''</center> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
| − | | | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''1'''</center> |
| − | | | + | | style="padding:0.079cm;"| <center>'''1900/01/01 to 2011/02/15'''</center> |
| − | | | + | | style="padding:0.079cm;"| <center>4H045</center> |
| − | | | + | | style="padding:0.079cm;"| <center>DA15+DA16+DA17+DA18 </center> |
| + | | style="padding:0.079cm;"| <center>1298</center> | ||
| + | |||
| + | |} | ||
| + | |||
| + | <br> | ||
| + | |||
| + | |||
| + | '''Total patents: 1298 (Relevancy ~10%)''' | ||
| + | |||
| + | |||
| + | * '''F-Terms and theme used in search''' | ||
| + | |||
| + | {|border="2" cellspacing="0" cellpadding="4" width="75%" | ||
| + | |align = "center" bgcolor = "#99CCFF" colspan = "3"|'''Japanese F-term search''' | ||
| + | |align = "center" bgcolor = "#99CCFF"|'''Definition''' | ||
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#C0C0C0"|'''Sr. No.''' |
| − | |align = "center"| | + | |align = "center"|'''F- Term theme''' |
| − | | | + | |align = "center"|'''4H045''' |
| − | | | + | |align = "center"|Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof |
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#C0C0C0"|'''1''' |
| − | |align = "center"| | + | |align = "center"|F-term |
| − | | | + | |align = "center"|DA15 |
| − | | | + | |align = "center"|Peptide or protein characterised by function - Interferons |
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#C0C0C0"|'''2''' |
| − | |align = "center"| | + | |align = "center"|F-term |
| − | | | + | |align = "center"|DA16 |
| − | | | + | |align = "center"|Alpha-interferons |
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#C0C0C0"|'''3''' |
| − | |align = "center"| | + | |align = "center"|F-term |
| − | | | + | |align = "center"|DA17 |
| − | | | + | |align = "center"|Beta-interferons |
|- | |- | ||
| − | |align = "center"| | + | |align = "center" bgcolor = "#C0C0C0"|'''4''' |
| − | |align = "center"| | + | |align = "center"|F-term |
| − | + | |align = "center"|DA18 | |
| − | + | |align = "center"|Gamma-interferons | |
| − | + | ||
| − | |align = "center"| | + | |
| − | |align = "center"| | + | |
| − | + | ||
| − | + | ||
|- | |- | ||
|} | |} | ||
| − | == | + | ===Scientific Literature Search=== |
| − | + | ||
| − | + | {|border="2" cellspacing="0" cellpadding="4" width="50%" | |
| − | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>S.No</center> | |
| − | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>Database</center> | |
| − | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>Query</center> | |
| − | {| border="2" cellspacing="0" cellpadding=" | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>Limits by Date</center> |
| − | | | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>No.Of Hits</center> |
| − | | | + | |
| − | | | + | |
| − | | | + | |
| − | | | + | |
| − | + | ||
|- | |- | ||
| − | | style="background-color:#99ccff"| <center> | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>1</center> |
| − | | style=" | + | | style="padding:0.079cm;"| <center>Pubmed</center> |
| + | | style="padding:0.079cm;"| <center>(Melanoma Or carcinoma or cancer* or tumor) And (IFN* OR Interferon)</center> | ||
| + | | style="padding:0.079cm;"| <center>20000101-20110221</center> | ||
| + | | style="padding:0.079cm;"| <center>28402</center> | ||
|- | |- | ||
| − | | style="background-color:#99ccff"| <center> | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>2</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>Scirus</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>(Melanoma Or carcinoma or cancer* or tumor) And (IFN* OR Interferon)</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>2000-2011</center> |
| − | | | + | | style="padding:0.079cm;"| <center>24835</center> |
| − | + | ||
| − | | | + | |
|- | |- | ||
| − | | style="background-color:#99ccff"| <center> | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>3</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>Google Scholar</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>(Melanoma Or carcinoma or cancer* or tumor) And (IFN* OR Interferon)</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>2000-2011</center> |
| − | | | + | | style="padding:0.079cm;"| <center>21100</center> |
| − | | | + | |
| − | | | + | |} |
| + | |||
| + | ==Sample patents== | ||
| + | |||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''S.No'''</center> | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''Patent/Publication No'''</center> | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''Date Of Publication'''</center> | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''Assignee'''</center> | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''Title'''</center> | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''Abstract'''</center> | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''Problem'''</center> | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''Solution'''</center> | ||
|- | |- | ||
| − | | style="background-color:#99ccff"| <center>''' | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''1'''</center> |
| − | | <center>[http:// | + | | style=";padding:0.079cm;"| <center>[http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=7482014.PN.&OS=PN/7482014&RS=PN/7482014 US7482014B2]</center> |
| − | | <center>2009</center> | + | | style=";padding:0.079cm;"| <center>01/27/2009</center> |
| − | | <center> | + | | style=";padding:0.079cm;"| <center>Schering Corporation</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Melanoma therapy </center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Methods for treating treatment-naive as well as treatment-experienced patients having melanoma to increase the progression-free survival time involving administering a therapeutically effective amount of pegylated interferon-alpha, e.g., preferably pegylated interferon alpha-2b, as adjuvant therapy to definitive surgery are disclosed.</center> |
| − | | The | + | | style=";padding:0.079cm;"| <center>The problem is with the treatment methods that are employed with previously employed dose regimens for treating Melanoma after definitive surgical removal of the lesions.This led to the occurance of hematologic, neurologic and constitutional toxicities.Subject compliance with the dosage and dosage regimen during both phases is considered to be important to achieve maximum clinical benefit. </center> |
| + | | style=";padding:0.079cm;"| <center>The higher patience compliance is achieved with the improved methods of treatment of melanoma.A therapeutically effective dose of pegylated interferon alpha for a time period sufficient to increase the progression-free survival time was administered to the patient.The treatment regimen includes a first dose of 6.0 micrograms/kg of PEG.sub.12000 interferon alpha-2b once a week for eight weeks, and then administering to the patient a second dose of 3.0 or less micrograms/kg of PEG.sub.12000 interferon alpha-2b once a week for the remainder of a five year treatment period. </center> | ||
|- | |- | ||
| − | | style="background-color:#99ccff"| <center>''' | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''2'''</center> |
| − | | <center>[http:// | + | | style=";padding:0.079cm;"| <center>[http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=5997858.PN.&OS=PN/5997858&RS=PN/5997858 US5997858A]</center> |
| − | | style=" | + | | style=";padding:0.079cm;"| <center>12/7/1999</center> |
| − | | <center> | + | | style=";padding:0.079cm;"| <center>Pharma Pacific Pty Ltd.</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Stimulation of host defense mechanisms against tumors</center> |
| − | + | | style=";padding:0.079cm;"| <center>A method for treating neoplastic disease in a mammal via administering to the mammal a therapeutically effective amount of an interferon via oromucosal contact. The amount of interferon administered is less than an amount which induces a pathological response when administered parenterally.</center> | |
| − | | | + | | style=";padding:0.079cm;"| <center>The problem is with the method employed for the treatment of neoplastic diseases.The administration of low doses of interferon as a nasal spray or as an oral liquid formulation in the treatment of the neoplastic diseases is not effective in the previous patents.There is no experimental evidence regarding the administration mode of the interferon,though it was anticipated that administrations through other modes is possible to deliver effectively and treating the same conditions.</center> |
| + | | style=";padding:0.079cm;"| <center>The solution to the problem is solved by first controlled study in an animal model of the efficacy of oromucosally administered interferon for the treatment of neoplastic diseases.The administration is done oromucosally in asingle dose by almost all forms of Interferons .the amount administered is from about 1500 IU to about 20.times.10.sup.6 IU for a 70 kg man per day.This amount is less than the amount that induces a pathological response in the mammal when administered parenterally.</center> | ||
|- | |- | ||
| − | | style="background-color:#99ccff"| <center>''' | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''3'''</center> |
| − | | <center>[http:// | + | | style=";padding:0.079cm;"| <center>[http://v3.espacenet.com/publicationDetails/biblio?DB=EPODOC&adjacent=true&locale=en_EP&FT=D&date=19881026&CC=EP&NR=0288055A1&KC=A1 EP288055A1]</center> |
| − | | <center> | + | | style=";padding:0.079cm;"| <center>10/26/1988</center> |
| − | | <center> | + | | style=";padding:0.079cm;"| <center>MERRELL DOW PHARMACEUTICALS INC.</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Use of ODC inhibitors, dacarbazine, and interferon, in the treatment of malignant melanoma </center> |
| − | + | | style=";padding:0.079cm;"| <center>This invention relates to the improvement of the polyamine depletion effects of ornithine decarboxylase inhibitors, the improvement being effected by the use of Interferon and Dacarbazine in conjunctive therapy with said inhibitors.</center> | |
| − | | | + | | style=";padding:0.079cm;"| <center>The problem in this patent is associated with the methods and drugs that are used for treating the pathological disease conditions such as cancer.Polyamines mechanism is not known and there are some evidences that ODC inhibitors may exert their therapeutic effect by blocking the formation of the polyamines and thereby slowing, interrupting, or arresting the proliferation and metastases of the tumor tissue. So certain methods are explored to find out the same kind of effect on treating cancers.</center> |
| + | | style=";padding:0.079cm;"| <center>The solution was found to be the improved methods in treating the cancer with the use of Interferon and Dacarbazine when these disease states are treated with irreversible inhibitors of ornithine decarboxylase.This includes a pharmaceutical product containing an ornithine decarboxylase inhibitor, Interferon and Dacarbazine as a combined preparation for simultaneous, separate or sequential use in treating rapidly-proliferating cell-growth disease states. Even the methods for the formulation are disussed in this patent.</center> | ||
|- | |- | ||
| − | | style="background-color:#99ccff"| <center>''' | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''4'''</center> |
| − | | <center>[http:// | + | | style=";padding:0.079cm;"| <center>[http://v3.espacenet.com/publicationDetails/biblio?DB=EPODOC&adjacent=true&locale=en_EP&FT=D&date=19871014&CC=EP&NR=0241242A1&KC=A1 EP241242A1]</center> |
| − | | <center> | + | | style=";padding:0.079cm;"| <center>10/14/1987</center> |
| − | | <center> | + | | style=";padding:0.079cm;"| <center>CETUS ONCOLOGY CORPORATION</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>The use of interferon-beta and interleukin-2 for combination therapy and compositions therefor </center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Anti-tumor activity in humans can be augmented by administering to the human patient and effective amount of IFN-β and IL-2 in combination. The composition of IFN-β and IL-2 may be prepared invitro or administered separately to the patient. The composition is useful for prophylactic or therapeutic treatment of such cancers as melanoma, colon cancer lung cancer and breast cancer.</center> |
| − | + | | style=";padding:0.079cm;"| <center>The problem in this patent is about the use of interferons seperately in treating the cancers.When administered seperately they were found to induce a response that was good.So an approach was thought of where the combination therapy was given to produce better results.</center> | |
| + | | style=";padding:0.079cm;"| <center>The concern of the prior art was addressed with the successful administration of a combination therapy with Interferon beta and interleukin-2 as an anti-tumor therapeutic or prophylactic agent. It was made suitable for administration to human patients for therapeutic or prophylactic treatment of cancer comprising formulating together, whether by mixing or providing separate doses.The administration is done parenterally.</center> | ||
|- | |- | ||
| − | | style="background-color:#99ccff"| <center>''' | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''5'''</center> |
| − | | <center>[http:// | + | | style=";padding:0.079cm;"| <center>[http://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220100086518%22.PGNR.&OS=DN/20100086518&RS=DN/20100086518 US20100086518] </center> |
| − | | <center> | + | | style=";padding:0.079cm;"| <center>4/8/2010</center> |
| − | | <center> | + | | style=";padding:0.079cm;"| <center>NOVARTIS AG</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Treatment of melanoma</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Methods of treating melanoma include administering a compound of Structure I, a tautomer of the compound, a pharmaceutically acceptable salt of the compound, a pharmaceutically acceptable salt or the tautomer, or a mixture thereof to a subject. The compound, tautomer, salt of the compound, salt of the tautomer, or mixture thereof may be used to prepare medicaments for treating metastatic cancer. The variable A has the values defined herein.</center> |
| − | | The | + | | style=";padding:0.079cm;"| <center>The problem is that though there are many methods of treating cancer , still there is a need for the advancements in the technologies to be adopted to arrive at better results.The compounds such as quinoline derivatives were used and were disclosed in the prior art for the treatment of Melanoma.The compounds that were used previously were found to be associated with the side effects.</center> |
| + | | style=";padding:0.079cm;"| <center><nowiki>The solution was found to be finding of compounds that can effectively administered for treating Melanoma.It relates to the use of compounds such as 4-amino-5-fluoro-3-[6-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]quino- lin-2(1H)-one and tautomers, salts, and mixtures thereof in treating melanoma and preparing medicaments for treating melanoma. The therapeutically effective amount of the compound can range from about 0.25 mg/kg to about 30 mg/kg body weight of the subject.</nowiki></center> | ||
|- | |- | ||
| − | | style="background-color:#99ccff"| <center>''' | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''6'''</center> |
| − | | <center>[http:// | + | | style=";padding:0.079cm;"| <center>[http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=4846782.PN.&OS=PN/4846782&RS=PN/4846782 US4846782] </center> |
| − | | <center> | + | | style=";padding:0.079cm;"| <center>7/11/1989</center> |
| − | | <center> | + | | style=";padding:0.079cm;"| <center>Schering Corporation</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Treatment of cancer with interferon and radiotherapy </center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Radiation sensitive human cancers are treated with combined interferon and radiation therapy.</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Radiation therapy emerged some years back for the treatment of cancers.It was observed that the results are good.But there was a need felt to effectively increase the efficacy of radiation treatment.So to develop radiation sensitizers or potentiators which enable the radiation to cause increased tumor destruction. Despite numerous laboratory and clinical studies, no single agent has, to date, emerged as the optimal radiation sensitizer. </center> |
| + | | style=";padding:0.079cm;"| <center>The problem could be addressed by an effective treatment means using administering subcutaneously to such patients between 2.0.times.10.sup.6 IU/m.sup.2 and 5.0.times.10.sup.6 IU/m.sup.2 of recombinant DNA-alpha-2-interferon .This is done three days a week at a time on those days prior to radiation therapy.The doses are from 15 to 35 Gy are administered five days a week including those days on which interferon is administered.</center> | ||
|- | |- | ||
| − | | style="background-color:#99ccff"| <center>''' | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''7'''</center> |
| − | | <center>[http:// | + | | style=";padding:0.079cm;"| <center>[http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=5824300.PN.&OS=PN/5824300&RS=PN/5824300 US5824300] </center> |
| − | | <center> | + | | style=";padding:0.079cm;"| <center>10/20/1998</center> |
| − | | <center> | + | | style=";padding:0.079cm;"| <center>The Texas A&M University System</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Treatment of neoplastic disease with oral interferon </center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Neoplastic diseases are treated by the administration of human interferon, particularly IFN-α, at a dosage of from about 0.01 to about 5 IU/lb./day such that the interferon is held in contact with the patient's oral and pharyngeal mucosae. The interferon is administered in a solid dosage from, e.g., a saliva-dissolvable lozenge.</center> |
| − | | The | + | | style=";padding:0.079cm;"| <center>Though the research is intensive in the field of interferons,there exists a substantial lack of uniformity in such matters as classification of interferon types. There are also numerous, sometimes contradictory, theories concerning the mode of action of interferon in producing clinical effects.It became apparent that exogenous interferon was sometimes capable of effecting regression or remission of various metastatic diseases. so different studies are conducted to know the clinical agent of choice for the prevention of cancers.</center> |
| + | | style=";padding:0.079cm;"| <center>The present invention is based on applicant's discovery that interferon can be used as a consistently effective therapeutic agent for treatment of diseases having an immunopathologic basis--characterized by inadequate immune response and persistence of the disease.The interferon is administered in an amount of about 0.01 to about 5 IU/lb of patient body weight per day. Multiple dose daily regimen is given to the patients.They aid in the better treatment of cancers.</center> | ||
|- | |- | ||
| − | | style="background-color:#99ccff"| <center>''' | + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''8'''</center> |
| − | | <center>[http:// | + | | style=";padding:0.079cm;"| <center>[http://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220020107184%22.PGNR.&OS=DN/20020107184&RS=DN/20020107184 US20020107184]</center> |
| − | | <center> | + | | style=";padding:0.079cm;"| <center>8/8/2002</center> |
| − | | <center> | + | | style=";padding:0.079cm;"| <center>None</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>METHOD FOR TREATING MELANOMA </center> |
| − | | | + | | style=";padding:0.079cm;"| <center>The present invention discloses a method for treating patients having melanoma or melanoma associated symptoms by parenterally administering Product R, a peptide-nucleic acid preparation.</center> |
| − | | | + | | style=";padding:0.079cm;"| <center>Melanomas are usually treated by surgical excision, while patients with thick melanomas and those with regional or distant metastasis may benefit from other forms of therapy.Cytokines have been tested in the treatment of different skin cancers during the last decade, and treatment schedules have been established or proposed for several malignant skin tumors. Preferentially, the interferons and interleukin-2 were found to be effective in treating skin cancers including melanoma.But they were needed to be checked in combination with other products as they were anticipated to yield better results.</center> |
| + | | style=";padding:0.079cm;"| <center>The new method of treatment using the product R in combination with interferons not only sounded effectively but also proved to be an effective means .The administration is done in an sterile injectible form.</center> | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''9'''</center> | ||
| + | | style=";padding:0.079cm;"| <center>[http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=4762705.PN.&OS=PN/4762705&RS=PN/4762705 US4762705] </center> | ||
| + | | style=";padding:0.079cm;"| <center>8/9/1988</center> | ||
| + | | style=";padding:0.079cm;"| <center>Schwimmer, Adolf W. | Schwartz, Irwin Steven | Rubin, David</center> | ||
| + | | style=";padding:0.079cm;"| <center>Cancer therapy with interferon </center> | ||
| + | | style=";padding:0.079cm;"| <center>The effectiveness of interferon for treatment against cancer may be increased by first administering an agent for inhibiting tyrosinase. In this manner the tyrosinase which is known to be produced by malignancies, and which may cause inactivation of the interferon, will be substantially inactivated prior to the interferon administration.</center> | ||
| + | | style=";padding:0.079cm;"| <center>Some of the the prior art patents doesn't trust on the use of interferons for treating all types of malignancies.The reason being the interferons are easily denatured in the enzymatic processes.So attempts were made out initially to find out the reasons for the denaturation even at high doses.Efforts were made to improve methods of cancer therapy using interferon.</center> | ||
| + | | style=";padding:0.079cm;"| <center>The solution has come out in the form of improved treatment method for treating cancer by the efforts of the present inventor.As the reason for the denaturation was found to be tyrosinase,attempts were made seriously to supress this tyrosinase.A composition was made finally with D-penicillamine that can suppress tyrosinase.</center> | ||
| + | |||
| + | |- | ||
| + | | style="background-color:#99ccff;;padding:0.079cm;"| <center>'''10'''</center> | ||
| + | | style=";padding:0.079cm;"| <center>[http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=5190751.PN.&OS=PN/5190751&RS=PN/5190751 US5190751] </center> | ||
| + | | style=";padding:0.079cm;"| <center>3/2/1993</center> | ||
| + | | style=";padding:0.079cm;"| <center>Schering Corporation</center> | ||
| + | | style=";padding:0.079cm;"| <center>Treatment of certain leukemias with a combination of gamma interferon and alpha interferon </center> | ||
| + | | style=";padding:0.079cm;"| <center>Human leukemia T-cells and B-cells are inhibited from proliferating by treatment with a combination of recombinant human alpha and gamma interferons, either simultaneously or sequentially, and the alpha interferon is preferably recombinant human alfa-2b interferon.</center> | ||
| + | | style=";padding:0.079cm;"| <center>The patent in the prior art posed a lot of problems with the use of gamma interferons alone in terms of the purity as the preparations previously were found to be contaminated.When used singly for the treatment of lekimias they were found to yield ineffective results.</center> | ||
| + | | style=";padding:0.079cm;"| <center>The solution was found to administer alpha and gamma interferons for the treatment of leukemias.It could inhibit the proliferation of susceptible leukemia cells with a cell proliferation inhibiting amount of a combination of both of the interferons.They are adminstered sequentially and simultaneously too to give good results.</center> | ||
|} | |} | ||
| − | + | ==Taxonomy== | |
| − | + | ||
| − | + | ||
| − | + | [[Image:taxonomy melanoma mod1.jpg|700px|center|thumb| '''Taxonomy''']] | |
| − | {|border="2" cellspacing="0" cellpadding="4" width=" | + | == Sample Analysis== |
| − | | style="background-color:#99ccff;"| <center> | + | * '''Patents:''' The above sample patents were analysed according to the taxonomy. |
| − | | style="background-color:#99ccff;"| <center> | + | [[Media:Sample analysis of Treating of melanoma.xls| '''Click here to download Sample analysis sheet on Interferon for treatment of Melanomas''']] |
| − | | style="background-color:#99ccff;"| <center> | + | |
| − | | style="background-color:#99ccff;"| <center> | + | * '''Scientific Literature:'''* [[Media:Article Analysis Of Melanoma.xls |'''Click here to download Melanoma Treatment Using IFN: sample articles]] |
| + | |||
| + | ==Patent Ranking== | ||
| + | 10 Sample Patents were ranked according to the patent focus. | ||
| + | *Patent Ranking Details | ||
| + | 1 : Granted Patent & Focus in Independent Claim <br> | ||
| + | 2 : Granted Patent & Focus in Dependent Claim <br> | ||
| + | 3 : Published Patent & Focus in independent Claim <br> | ||
| + | 4 : Published Patent & Focus in Dependent claim <br> | ||
| + | |||
| + | {|border="2" cellspacing="0" cellpadding="4" width="50%" | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>S.No</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>Patent</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>Type</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>Patent Ranking</center> | ||
|- | |- | ||
| − | | style="background-color:#99ccff;"| <center> | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>1</center> |
| − | | | + | | style="padding:0.079cm;"| <center>US7482014B2</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Granted And Independent Claim</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>1</center> |
|- | |- | ||
| − | | style="background-color:#99ccff;"| <center> | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>2</center> |
| − | | | + | | style="padding:0.079cm;"| <center>US5997858A</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Granted And Independent Claim</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>1</center> |
|- | |- | ||
| − | | style="background-color:#99ccff;"| <center> | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>3</center> |
| − | | | + | | style="padding:0.079cm;"| <center>EP288055A1</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Granted And Independent Claim</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>1</center> |
|- | |- | ||
| − | | style="background-color:#99ccff;"| <center> | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>4</center> |
| − | | | + | | style="padding:0.079cm;"| <center>EP241242A1</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Granted And Dependent Claim</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>2</center> |
|- | |- | ||
| − | | style="background-color:#99ccff;"| <center> | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>5</center> |
| − | | | + | | style="padding:0.079cm;"| <center>US20100086518 </center> |
| − | | | + | | style="padding:0.079cm;"| <center>Published And Dependent</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>4</center> |
|- | |- | ||
| − | | style="background-color:#99ccff;"| <center> | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>6</center> |
| − | | | + | | style="padding:0.079cm;"| <center>US4846782 </center> |
| − | | | + | | style="padding:0.079cm;"| <center>Granted And Dependent Claim</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>2</center> |
|- | |- | ||
| − | | style="background-color:#99ccff;"| <center> | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>7</center> |
| − | | | + | | style="padding:0.079cm;"| <center>US5824300 </center> |
| − | | | + | | style="padding:0.079cm;"| <center>Granted And Dependent Claim</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>2</center> |
|- | |- | ||
| − | | style="background-color:#99ccff;"| <center> | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>8</center> |
| − | | | + | | style="padding:0.079cm;"| <center>US20020107184</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Published And Independent</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>3</center> |
|- | |- | ||
| − | | style="background-color:#99ccff;"| <center> | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>9</center> |
| − | | | + | | style="padding:0.079cm;"| <center>US4762705 </center> |
| − | | | + | | style="padding:0.079cm;"| <center>Granted And Independent Claim</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>1</center> |
|- | |- | ||
| − | | style="background-color:#99ccff;"| <center> | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>10</center> |
| − | | | + | | style="padding:0.079cm;"| <center>US5190751 </center> |
| − | | | + | | style="padding:0.079cm;"| <center>Granted And Independent Claim</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>1</center> |
|} | |} | ||
| − | == | + | ==Clinical Trials== |
| + | *'''Database:''' [http://clinicaltrials.gov/ '''Clinical trials'''] | ||
| + | *'''Searched on:''' Feb 25th, 2011 | ||
| + | |||
| + | *[[Media:clinical trials treatment of melanoma.xls |'''Please click here to download the clinical trial excel sheet''']] | ||
| − | + | ==IP Activity Graphs Of Sample Patents== | |
| − | + | ===IP activity based on priority years=== | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
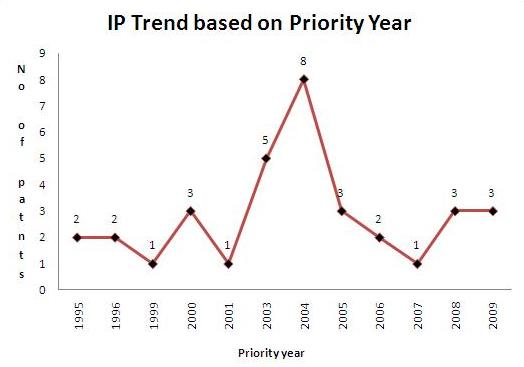
| − | + | * Total of 10 Sample patents(basic patent number) were taken into consideration for the IP activity based on priority years. | |
| − | + | [[Image:IP act PS priority year Treatment Of Melanoma1.jpg|center|thumb|800 px| IP activity based on priority years]] | |
| − | + | ||
| − | + | ||
| − | | | + | |
| − | | | + | |
| − | + | ===IP activity based on publication years=== | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
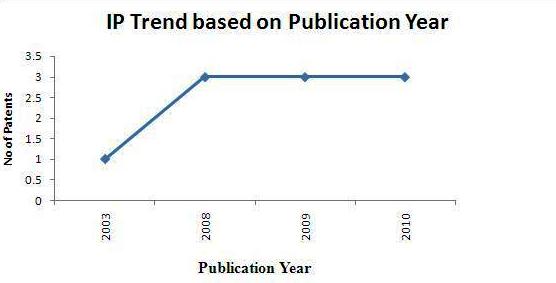
| − | + | * Total of 10 Sample patents(basic patent number) were taken into consideration for the IP activity based on publication years. | |
| − | + | [[Image:IP act PS publication year Treatment Of Melanoma1.jpg|center|thumb|800 px| IP activity based on publication years]] | |
| − | + | ||
| − | | | + | |
| − | | | + | |
| − | | | + | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | ===Geographical Distribution based on family members=== | |
| − | + | ||
| − | + | ||
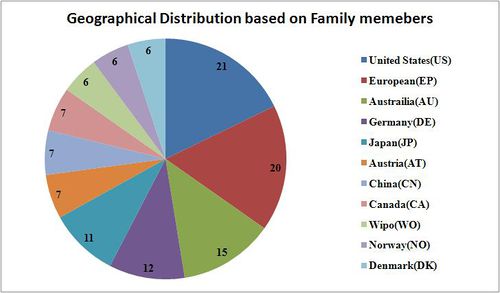
| − | + | * The geographical distribution is based on 10 sample patent numbers along with all their family members. | |
| + | [[Image:Geographical Distribution based on Family members Melanoma 1.jpg|center|thumb|500 px| Geographical Distribution based on Family members Melanoma]] | ||
| − | {| border="2" cellspacing="0" cellpadding=" | + | ==Market Report== |
| − | | style="background-color:# | + | ===Interferon types & Their Compositions=== |
| − | | style="background-color:# | + | {|border="2" cellspacing="0" cellpadding="4" width="100%" |
| − | | style="background-color:# | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''S.No'''</center> |
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Generic Name'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Brand Name'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Company Name'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Composition'''</center> | ||
|- | |- | ||
| − | | style="background-color:# | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''1'''</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Alpha IFN</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Intron®,Roferon®-A</center> |
| + | | style="padding:0.079cm;"| <center>Schering Corporation</center> | ||
| + | | style="padding:0.079cm;"| <center>Active Ingredient-Interferon alfa-2aInactive Ingredients- sodium chloride, ammonium acetate, polysorbate 80, glycine, sodium phosphate dibasic,sodium phosphate monobasic, human albumin, preservative: benzyl alcohol.</center> | ||
|- | |- | ||
| − | | style="background-color:# | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''2'''</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Beta IFN</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Avonex</center> |
| + | | style="padding:0.079cm;"| <center>Biogen IDEC</center> | ||
| + | | style="padding:0.079cm;"| <center>Active Ingredient-Beta interferon,Inactive Ingredients-65 to 90 wt % of polyol,and a p-hydroxybenzoate,carboxymethyl cellulose,human serum albumin</center> | ||
|- | |- | ||
| − | | style="background-color:# | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''3'''</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Gamma IFN</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Actimmune®</center> |
| + | | style="padding:0.079cm;"| <center>Intermune</center> | ||
| + | | style="padding:0.079cm;"| <center>Active Ingredient-interferon gamma-1b.,Inactive Ingredients-Polyethylene Glycol,dextran ,hydroxyethylstarch </center> | ||
|- | |- | ||
| − | | style="background-color:# | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''4'''</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Pegylated IFN</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Peg Intron</center> |
| + | | style="padding:0.079cm;"| <center>Schering-Plough</center> | ||
| + | | style="padding:0.079cm;"| <center>Active ingredient-peginterferon alfa-2b,Inactive ingredients: dibasic sodium phosphate anhydrous, monobasicsodium phosphate dihydrate, sucrose, polysorbate 80.</center> | ||
|- | |- | ||
| − | | style="background-color:# | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''5'''</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Recombinant IFN</center> |
| − | | | + | | style="padding:0.079cm;"| <center>(Rebetron®, Rebetol®).</center> |
| + | | style="padding:0.079cm;"| <center>Schering Corporation</center> | ||
| + | | style="padding:0.079cm;"| <center>Active Ingredient-Ribavirin,Inactive Ingredients-microcrystalline cellulose, lactose monohydrate, croscarmellose sodium,sodium phosphate dibasic and sodium phosphate dibasic and sodium phosphate monobasic as buffering agents;human albumin as a stabilizer.</center> | ||
| + | |||
| + | |} | ||
| + | |||
| + | ===Interferon Types & Description Of Products=== | ||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''S.No'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Company Name'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Product'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Description'''</center> | ||
| + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Source'''</center> | ||
|- | |- | ||
| − | | style="background-color:# | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''1'''</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Schering Corporation</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Intron®,Roferon®-A</center> |
| + | | style="padding:0.079cm;"| <center>Intron A is an interferon, a group of naturally occurring proteins that were first discovered as a result of their ability to prevent viral replication. Intron A is marketed in 72 countries worldwide for as many as 16 indications.In the United States it has been cleared for use by the FDA for chronic viral hepatitis B, chronic viral hepatitis C, malignant melanoma, hairy cell leukemia, AIDS-related Kaposi’s sarcoma and condylomata acuminata (venereal warts).INTRON A recombinant for Injection has been classified as an alpha interfero nand is a water-soluble protein with a molecular weight of 19,271 daltons produced by recombinant DNA techniques. It is obtained from the bacterial fermentation of a strain of Escherichia coli bearing a genetically engineered plasmid containing an interferon alfa- 2b gene from human leukocytes. The fermentation is carried out in a defined nutrient medium containing the antibiotic tetracycline hydrochloride at a concentration of 5 to 10 mg/L; the presence of this antibiotic is not detectable in the final product. The specific activity of interferon alfa-2b, recombinant is approximately 2.6 x 108 IU/mg protein as measured by the HPLC assay.</center> | ||
| + | | style="padding:0.079cm;"| <center>[http://www.introna.com/maintenance.html http://www.introna.com/maintenance.html]</center> | ||
|- | |- | ||
| − | | style="background-color:# | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''2'''</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Biogen IDEC</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Avonex</center> |
| + | | style="padding:0.079cm;"| <center>Avonex, manufactured by Biogen, is a form of beta interferon (interferon beta, IFN-b) used to modify the course of multiple sclerosis. While not a cure, Avonex has been shown in clinical trials to reduce the average relapse rate in people with the relapsing-remitting multiple sclerosis form of the disease. It is identical to the naturally occurring protein found in the human body. It is manufactured by extracting the drug from Chinese hamster ovary cells. Avonex is the same substance as Rebif but administered differently (30 mcg, intra-muscularly, once a week as against 22 mcg or 44 mcg, sub-cutaneously, 3 times a week for Rebif). Avonex is usually given in the large muscles of the thigh, upper arm, or hip.</center> | ||
| + | | style="padding:0.079cm;"| <center>[http://www.mult-sclerosis.org/Avonex.html http://www.mult-sclerosis.org/Avonex.html]</center> | ||
|- | |- | ||
| − | | style="background-color:# | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''3'''</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Intermune</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Actimmune®</center> |
| + | | style="padding:0.079cm;"| <center>Actimmune(R) is a synthesized version of interferon gamma, a naturally occurring protein believed to stimulate the immune system. InterMune markets Actimmune(R) for the treatment of two life-threatening congenital diseases: chronic granulomatous disease and severe, malignant osteopetrosis. The most common side effects are flu-like symptoms, including headache, fatigue, fever, chills, and rash. InterMune was granted two composition-of-matter patents related to interferon gamma-1b in the United States, extending its patent protection until 2022.</center> | ||
| + | | style="padding:0.079cm;"| <center>[http://www.actimmune.com/ http://www.actimmune.com/]</center> | ||
|- | |- | ||
| − | | style="background-color:# | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''4'''</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Schering-Plough</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Peg Intron</center> |
| + | | style="padding:0.079cm;"| <center>Peg-Intron (peginterferon alfa-2b) Powder for Injection has been approved by the FDA.Peg-Intron is a longer-acting formulation of Schering-Plough's Intron A, which is a recombinant version of a naturally occurring alpha interferon. In contrast to Intron A, which is administered three times weekly, Peg-Intron is administered subcutaneously once a week. This reduced frequency of administration may increase patient compliance.</center> | ||
| + | | style="padding:0.079cm;"| <center>[http://www.pegintron.com/index.html http://www.pegintron.com/index.html]</center> | ||
|- | |- | ||
| − | | style="background-color:# | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''5'''</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Schering Corporation</center> |
| − | | <center> | + | | style="padding:0.079cm;"| <center>(Rebetron®, Rebetol®).</center> |
| + | | style="padding:0.079cm;"| <center>REBETOL is a medicine used with either interferon alfa-2b (Intron A) or peginterferon alfa-2b (PegIntron) to treat chronic (lasting a long time) hepatitis C infection in people 3 years and older with liver disease.REBETOL Capsules consist of a white powder in a white, opaque, gelatin capsule. Each capsule contains 200 mg ribavirin and the inactive ingredients microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, and magnesium stearate. The capsule shell consists of gelatin, sodium lauryl sulfate, silicon dioxide, and titanium dioxide. The capsule is printed with edible blue pharmaceutical ink which is made of shellac, anhydrous ethyl alcohol, isopropyl alcohol, n-butyl alcohol, propylene glycol, ammonium hydroxide, and FD&C Blue #2 aluminum lake.</center> | ||
| + | | style="padding:0.079cm;"| <center>[http://www.merck.com/product/home.html http://www.merck.com/product/home.html]</center> | ||
|} | |} | ||
| − | |||
| − | + | ===Global Revenue Data Of products=== | |
| − | == | + | <table cellpadding="3" cellspacing="0"> |
| − | + | <tr> | |
| − | + | <td colspan="1" rowspan="2" style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thick solid rgb(0, 0, 0); border-left: thick solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>S.No</strong></td> | |
| − | + | <td colspan="1" rowspan="2" style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>Generic Name</strong></td> | |
| − | + | <td colspan="1" rowspan="2" style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>Brand Name</strong></td> | |
| + | <td colspan="1" rowspan="2" style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>Company</strong></td> | ||
| + | <td colspan="3" rowspan="1" style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>Global revenue ($ Million )</strong></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>2008</strong></td> | ||
| + | <td style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>2009</strong></td> | ||
| + | <td style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0); border-right: thick solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>2010</strong></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thick solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>1</strong></td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Alpha IFN</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Intron®,Roferon®-A</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Schering Corporation</td> | ||
| + | <td style="border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);"></td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="bottom">38.4</td> | ||
| + | <td style="border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0); border-right: thick solid rgb(0, 0, 0);"></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thick solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>2</strong></td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Beta IFN</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Avonex</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center"> Biogen IDEC</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">2518.4</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">2322.9</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0); border-right: thick solid rgb(0, 0, 0);" align="center" valign="center">2202.6</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thick solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>3</strong></td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Gamma IFN</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Actimmune®</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Intermune</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">29880</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">25428</td> | ||
| + | <td style="border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0); border-right: thick solid rgb(0, 0, 0);"></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thick solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>4</strong></td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Pegylated IFN</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Peg Intron</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center"> Schering-Plough</td> | ||
| + | <td style="border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);"></td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">148.7</td> | ||
| + | <td style="border-top: thin solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0); border-right: thick solid rgb(0, 0, 0);"></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="background: none repeat scroll 0% 0% rgb(153, 204, 255); font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-bottom: thick solid rgb(0, 0, 0); border-left: thick solid rgb(0, 0, 0);" align="center" bgcolor="#99ccff" valign="center"><strong>5</strong></td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-bottom: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Recombinant IFN</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-bottom: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">(Rebetron®, Rebetol®).</td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-bottom: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">Schering Corporation</td> | ||
| + | <td style="border-top: thin solid rgb(0, 0, 0); border-bottom: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);"></td> | ||
| + | <td style="font-size: 10pt; border-top: thin solid rgb(0, 0, 0); border-bottom: thick solid rgb(0, 0, 0); border-left: thin solid rgb(0, 0, 0);" align="center" valign="center">36.1</td> | ||
| + | <td style="border-width: thin thick thick thin; border-style: solid; border-color: rgb(0, 0, 0);"></td> | ||
| + | </tr> | ||
| + | </table> | ||
| − | == | + | ==Biosimilars Of Interferons== |
| − | + | ||
| − | + | Biosimilar interferons are the major drug class in the recombinant non-glycosylated proteins market due to their extensive use in the treatment of various genetic and environmental disorders. The overall biosimilar market for interferons is categorized into the submarkets for alpha, beta, and gamma interferons, all of which are used extensively in the treatment of various conditions such as lymphoma, hairy cell leukemia, and multiple sclerosis. | |
| − | + | ||
| − | + | While the interferon market growth may be inhibited by the side effects of each of its three drug categories, the market presents many opportunities to new entrants as only a few players currently operate in this relatively unfragmented market. | |
| − | + | The global recombinant interferon market stood at $75.3 million in 2008 and is expected to reach $3.9 billion by 2014 at a CAGR of 82.9% from 2009 to 2014. The American biosimilar interferons market is expected to attain a market worth $1.5 billion by 2014 at a CAGR of 91.5%.[http://goliath.ecnext.com/coms2/gi_0198-669040/4-Biosimilar-products.html Biosimilars ; Global Market] | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | |
| − | + | ||
| − | {| border="2" cellspacing="0" cellpadding="4" | + | |
| style="background-color:#99ccff;padding:0.079cm;"| <center>'''S.No'''</center> | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''S.No'''</center> | ||
| − | | style="background-color:#99ccff;padding:0.079cm;"| <center>''' | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Product'''</center> |
| − | | | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''Global market For Biosimilars'''</center> |
| + | |||
|- | |- | ||
| − | | style="padding:0.079cm;"| <center>1</center> | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''1'''</center> |
| − | | style="padding:0.079cm;"| <center> | + | | style="padding:0.079cm;"| <center>Interferon Alfa</center> |
| − | | | + | | style="padding:0.079cm;"| <center>The global market for biosimilar interferon Alfa was $36.7 million in 2008 and is expected to reach $1.5 billion in 2014 at a CAGR of 76.5% from 2009 to 2014. While Asian market for biosimilar Alfa interferons commanded the highest share in 2008 with $17.5 million, the American market is expected to have the highest CAGR of 88.3% from 2009 to 2014.The world's top two branded Interferon products generated sales of $1,510 million and $910 million each in 2008. The blockbuster sales of innovative Interferon Alfa products thus present encouragement for biosimilar Interferon Alfa manufacturers[http://goliath.ecnext.com/coms2/gi_0198-669040/4-Biosimilar-products.html data].</center> |
| + | |||
|- | |- | ||
| − | | style="padding:0.079cm;"| <center>2</center> | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''2'''</center> |
| − | | style="padding:0.079cm;"| <center> | + | | style="padding:0.079cm;"| <center>Interferon beta</center> |
| − | | | + | | style="padding:0.079cm;"| <center>The global biosimilar interferon beta-1a market is estimated to grow from $36.3 million in 2008 to $2.2 billion by 2014 at a CAGR of 87.8% from 2009 to 2014. However, the U.S. and Europe sales of branded interferon-betas are expected to fall from $4.6 billion in 2010 to $2.4 billion in 2017. The soaring cost of clinical development, the conservative prescribing habits of neurologists, and the expected decline in the use of interferon-betas may induce biosimilar developers to explore other classes of biologics to invest their R&D funds[http://goliath.ecnext.com/coms2/gi_0198-669040/4-Biosimilar-products.html data].</center> |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
| − | | | + | | style="background-color:#99ccff;padding:0.079cm;"| <center>'''3'''</center> |
| − | | | + | | style="padding:0.079cm;"| <center>Interferon gamma</center> |
| − | | | + | | style="padding:0.079cm;"| <center>The global biosimilar interferon gamma market was $2.2 million 2008 and is expected to reach $141.5 million in 2014 at a CAGR of 94.2% from 2009 to 2014. While the Asian market accounted for the highest share of $0.5 million in 2008, the American market is expected to have the highest CAGR of 107.6% from 2009 to 2014[http://goliath.ecnext.com/coms2/gi_0198-669040/4-Biosimilar-products.html data].</center> |
| − | | | + | |
| − | + | ||
| − | | | + | |
| − | | | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|} | |} | ||
Revision as of 23:13, 16 June 2011
Contents
- 1 Dashboard
- 2 Objective
- 3 Overview
- 4 Interactive Taxonomy
- 5 Concept Table
- 6 Intellectual property
- 6.1 Search strategy and concept
- 6.2 Search in Micropatent full text - English language search
- 6.3 Search in Micropatent full text - Foreign language search
- 6.4 Search in Micropatent MPI-INPADOC - English language search
- 6.5 Search in Micropatent MPI-INPADOC - Foreign language search
- 6.6 Search in Japanese database
- 6.7 Scientific Literature Search
- 7 Sample patents
- 8 Taxonomy
- 9 Sample Analysis
- 10 Patent Ranking
- 11 Clinical Trials
- 12 IP Activity Graphs Of Sample Patents
- 13 Market Report
- 14 Biosimilars Of Interferons
Dashboard
- Click here to view Sample Dashboard
NOTE: You need to install Internet Explorer 8.0 and Adobe Flash Player to view the Dashboard.
Please download Internet Explorer 8.0 and Adobe Flash player
Objective
Primary objective of the study was to perform a prior art search on usage of interferon for the treatment of melanoma.
To achieve our objective we performed following steps:
- Created a multi level taxonomy to categorize the patents using interferon for melanoma treatment
- Marked out relevant IPC, ECLA, US classes and Japanese F-term available for technology in question.
- Identified and clubbed relevant keywords with classes to extract relevant patents.
- Checked for patents in US, EP, PCT, JP, Great Britain, and German patent records
- Performed MPI-INPADOC search which cover bibliographic data for 71 countries and legal status for 42 countries
- Analyzed the patents and prepared an IPmap covering relevant patents for client usage.
Overview
Interferon
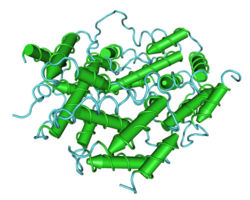
Interferons (IFNs) are natural cell-signaling proteins produced by the cells of the immune system of most vertebrates in response to challenges such as viruses, parasites and tumor cells. They belong to the large class of glycoproteins known as cytokines and are produced by a wide variety of cells in response to the presence of double-stranded RNA, a key indicator of viral infection. Source
Interferons assist the immune response by inhibiting viral replication within host cells, activating natural killer cells and macrophages, increasing antigen presentation to T lymphocytes, and increasing the resistance of host cells to viral infection. There are 3 known classes of interferons; type I, type II and type III. All classes are very important in fighting viral infections. Recent studies have shown that Interferon can also help stop the growth and spread of cancer cells. Source
Melanoma
Melanoma is the most serious type of skin cancer. It begins in skin cells called melanocytes. Melanocytes are the cells that make melanin, which gives skin its color. Melanin also protects the deeper layers of the skin from the sun's harmful ultraviolet (UV) rays.When people spend time in the sunlight, the melanocytes make more melanin and cause the skin to tan. This also happens when skin is exposed to other forms of ultraviolet light (such as in a tanning booth). If the skin receives too much ultraviolet light, the melanocytes may begin to grow abnormally and become cancerous. This condition is called melanoma.People with melanoma who have one or more positive lymph nodes are at a high risk to have their melanoma recur. It is believed that 70 to 80% of these individuals will have their melanoma come back within the next three to five years. Source
Interferon for treatment of melanoma
Over the past several decades, the incidence of melanoma has increased at a faster rate than that of any other solid tumor. In the 1930s, the lifetime risk for a person living in the U.S. to develop melanoma was 1 in 1,500. Currently, that risk is 1 in 74, and for 2003 it was estimated that 51,400 cases of invasive melanoma would be diagnosed. While efforts to improve early diagnosis through education have resulted in the increased detection of early-stage melanoma, many patients still present with high-risk primary melanomas.
A beacon of hope in the treatment of melanoma has long been the observation that melanoma is susceptible to attack by the host’s immune system. This has resulted in the testing of a remarkably broad spectrum of immunotherapies, including the use of nonspecific immunostimulants, various approaches to vaccine therapies, and cytokine therapy. Many of these approaches failed to demonstrate a significant clinical impact, and the practitioner had been left with few options in treating high-risk melanoma patients with adjuvant therapy. One exception to this, however, has been the use of adjuvant interferon alpha (IFN-{alpha})
While the precise mechanism of action remains poorly understood, there are multiple antitumor effects of IFN-{alpha}. These include a direct antiproliferative effect, the enhancement of natural killer cell activity, and the upregulation of tumor antigens and/or HLA class I and class II antigens. Initial phase II clinical studies with IFN-{alpha} in metastatic melanoma showed response rates in the 10%–20% range [4, 5]. These response rates, while encouraging, were not significant enough to lead to its widespread use in the treatment of metastatic melanoma. Source
Interactive Taxonomy
Concept Table
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
French Keywords Concept table
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
German Keywords Concept Table
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
Class codes identified for searches
- Relevant IPC classes
| IPC | |||
| Sr. No. | Class Code | Class definition | Class coverage |
| 1 | A61K003819 | Medicinal preparations containing peptides - Cytokines; Lymphokines; Interferons | Broad |
| 2 | A61K003821 | Medicinal preparations containing peptides Interferon | Specific |
| 3 | C07K001452 | Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof - Cytokines; Lymphokines; Interferons | Broad |
| 4 | C07K014555 | Peptides having more than 20 amino acids - Interferon | Specific |
| 5 | C07K001456 | Peptides having more than 20 amino acids - IFN-alpha | Specific |
| 6 | C07K014565 | Peptides having more than 20 amino acids - IFN-beta | Specific |
| 7 | C07K001457 | Peptides having more than 20 amino acids - IFN-gamma | Specific |
| 8 | A61P003500 | Therapeutic activity of chemical compounds or medicinal preparations -antineoplastic agents | Broad |
- Relevant ECLA classes
| ECLA | |||
| Sr. No. | Class Code | Class definition | Class coverage |
| 1 | A61K003819 | Medicinal preparations containing peptides - Cytokines; Lymphokines; Interferons | Broad |
| 2 | A61K003821 | Medicinal preparations containing Interferon | Specific |
| 3 | A61K38/21A | Medicinal preparations containing IFN-alpha | Specific |
| 4 | A61K38/21B | Medicinal preparations containing IFN-beta | Specific |
| 5 | A61K38/21C | Medicinal preparations containing IFN-gamma | Specific |
| 6 | C07K001452 | Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof - Cytokines; Lymphokines; Interferons | Broad |
| 7 | C07K014555 | Peptides having more than 20 amino acids - Interferon | Specific |
| 8 | C07K001456 | Peptides having more than 20 amino acids - IFN-alpha | Specific |
| 9 | C07K014565 | Peptides having more than 20 amino acids - IFN-beta | Specific |
| 10 | C07K001457 | Peptides having more than 20 amino acids - IFN-gamma | Specific |
| 11 | C07K014715G | Receptors; Cell surface antigens; Cell surface determinants - for interferons - | Specific |
- Relevant US classes
| US class | ||
| Sr. No. | Class Code | Class definition |
| 1 | 4240854 | DRUG, BIO-AFFECTING AND BODY TREATING COMPOSITIONS - this subclass provides for patents which broadly claim interferon or a method of treatment of interferon where the classification of the interferon as alpha, beta or gamma interferon is impossible |
| 2 | 4242811 | DRUG, BIO-AFFCTING AND BODY TREATING COMPOSITIONS - Virus (e.g., interferon-inducing virus, etc.) |
| 3 | 42400141 | DRUG, BIO-AFFECTING AND BODY TREATING COMPOSITIONS - Attached to lymphokine, cytokine, or other secreted growth regulatory factor, differentiation factor, or intercellular mediator specific for a hematopoietic cell (e.g., interferon, interleukin, macrophage factor, colony stimulating factor, erythropoietin); derivative thereof |
| 4 | 514889 | DRUG, BIO-AFFECTING AND BODY TREATING COMPOSITIONS - INTERFERON INDUCER |
| 5 | 530351 | CHEMISTRY: NATURAL RESINS OR DERIVATIVES; PEPTIDES OR PROTEINS; LIGNINS OR REACTION PRODUCTS THEREOF - Lymphokines, e.g., interferons, interlukins, etc. |
| 6 | 930142 | PEPTIDE OR PROTEIN SEQUENCE - Interferon |
| 7 | 4240851 | LYMPHOKINE - Included in this and the indented subclasses interferon, interleukin and macrophage factors (monokines) |
| 8 | 4240855 | Gamma or immune: This subclass is indented under subclass 85.4. Subject matter in which the interferon is gamma or immune interferon. |
| 9 | 4240856 | Subject matter in which the interferon is beta or fibroblast interferon. |
| 10 | 4240857 | Subject matter in which the interferon is alpha or leukocyte interferon. |
Intellectual property
Search strategy and concept
Date of Search: 1836 to Feb 3rd, 2011 Database used: Micropatent - Include extensive full text and MPI-Inpadoc searches
Search in Micropatent full text - English language search
Micro patent full text search allow search in fulltext of US, EP, PCT, Great Britain, and German patent records as well as the front page of JP documents. US, EP, and DE are covered at first publication and when granted.
| S. No. | Search concept | Search Scope | Search reason | Class Code (IPC,US,ECLA) | Search query | No. of hits |
| 1 | Interferon for treating Melanoma | Title, Abstract and Claims | Specific classes of interferon AND melanoma keywords | A61K003821* OR C07K014555 OR C07K001456 OR C07K014565 OR C07K001457 OR C07K014715G OR 4240854 OR 4242811 OR 42400141 OR 514889 OR 530351 OR 930142 | (Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte* NEAR3 (cancer OR carcinoma OR tumor))) | 576 |
| 2 | Interferon for treating Melanoma | Title, Abstract and Claims | Broad classes of interferon AND melanoma, interferon keywords | A61K003819 OR C07K001452 OR 4240851 OR 4240855 OR 4240856 OR 4240857 OR A61P003500 | (Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte* NEAR3 (cancer OR carcinoma OR tumor))) | 756 |
| 3 | Final query | 1 OR 2 | 1019 records 571 unique records | |||
Search in Micropatent full text - Foreign language search
Micro patent full text search allow search in fulltext of US, EP, PCT, Great Britain, and German patent records as well as the front page of JP documents. US, EP, and DE are covered at first publication and when granted.
| Sr. No. | Search concept | Language | Search Scope | Search reason | Class Code (IPC, ECLA) | Search query | No. of hits |
| 1 | Interferon for treating Melanoma | French | Title, Abstract and Claims | Specific classes of interferon AND melanoma’s foregin langugae keywords | A61K003821* OR C07K014555 OR C07K001456 OR C07K014565 OR C07K001457 OR C07K014715G | (mélanome or (Peau NEAR3 (Cancer or Carcinome or Tumeur)) OR (Mélanocytes Near3 (Cancer or Carcinome or Tumeur))) | 184 hits |
| German | (Melanom or (Haut NEAR3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst)) OR (Melanozyten Near3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst))) | ||||||
| 2 | Interferon for treating Melanoma | French | Title, Abstract and Claims | Broad classes of interferon AND melanoma’s and interferon’s foregin langugae keywords | A61K003819 OR C07K001452 OR A61P003500 | (mélanome or (Peau NEAR3 (Cancer or Carcinome or Tumeur)) OR (Mélanocytes Near3 (Cancer or Carcinome or Tumeur))) | 3375 hits |
| German | (Melanom or (Haut NEAR3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst)) OR (Melanozyten Near3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst))) | ||||||
| 3 | Final query | 1 OR 2 | 3422 hits (2023 unique records, 30-35 % relevant) | ||||
Search in Micropatent MPI-INPADOC - English language search
Micrpatent MPI-INPADOC search bibliographic data for 71 countries and legal status for 42. Only those patents were analyzed which have English title and/or abstract.
| Sr. No. | Search concept | Search Scope | Search reason | Class search | Search query | No. of hits |
| 1 | Interferon for treating Melanoma | Title and Abstract | Specific IPC classes of interferon AND melanoma keywords | A61K03821 OR C07K014555 OR C07K01456 OR C07K014565 OR C07K01457 | (Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte* NEAR3 (cancer OR carcinoma OR tumor))) | 174 |
| 2 | Interferon for treating Melanoma | Title and Abstract | Broad IPC classes of interferon AND melanoma, interferon keywords | A61K03819 OR C07K01452 OR A61P03500 | (IFN* OR *IFN OR interferon* OR *interferon OR huIFN) AND (Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte* NEAR3 (cancer OR carcinoma OR tumor))) | 484 |
| 3 | Interferon for treating Melanoma | Title and Abstract | Specific ECLA classes of interferon AND melanoma keywords | A61K03821* OR C07K014555 OR C07K01456 OR C07K014565 OR C07K01457 OR C07K014715G | (Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte* NEAR3 (cancer OR carcinoma OR tumor))) | 102 |
| 4 | Interferon for treating Melanoma | Title and Abstract | Broad ECLA classes of interferon AND melanoma, interferon keywords | A61K03819 OR C07K01452 OR A61P03500 | (IFN* OR *IFN OR interferon* OR *interferon OR huIFN) AND (Melanoma OR (Skin NEAR3 (cancer OR carcinoma OR tumor)) OR (Melanocyte* NEAR3 (cancer OR carcinoma OR tumor))) | 9 |
| 5 | Final query | 1 OR 2 OR 3 OR 4 | 587 hits 232 unique records | |||
Search in Micropatent MPI-INPADOC - Foreign language search
Micrpatent MPI-INPADOC search bibliographic data for 71 countries and legal status for 42. Only those patents were analyzed which have English title and/or abstract.
| Sr. No. | Search concept | Language | Search Scope | Search reason | Class Code (IPC, ECLA) | Search query | No. of hits |
| 1 | Interferon for treating Melanoma | French | Title and Abstract | Specific IPC/ECLA classes of interferon AND melanoma keywords | A61K03821 OR C07K014555 OR C07K01456 OR C07K014565 OR C07K01457 | (mélanome or (Peau NEAR3 (Cancer or Carcinome or Tumeur)) OR (Mélanocytes Near3 (Cancer or Carcinome or Tumeur))) | 4 hits |
| German | (Melanom or (Haut NEAR3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst)) OR (Melanozyten Near3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst))) | ||||||
| 2 | Interferon for treating Melanoma | French | Title and Abstract | Broad IPC classes of interferon AND melanoma, interferon keywords | A61K03819 OR C07K01452 OR A61P03500 | (IFN* OR *IFN OR Interféron* OR *Interféron OR huIFN) AND (mélanome or (Peau NEAR3 (Cancer or Carcinome or Tumeur)) OR (Mélanocytes Near3 (Cancer or Carcinome or Tumeur))) | 25 hits |
| German | (IFN* OR *IFN OR interferon* OR *interferon OR huIFN AND (Melanom or (Haut NEAR3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst)) OR (Melanozyten Near3 (Krebs or Karzinom or Krebsgeschwür or Tumor or Geschwulst))) | ||||||
| 3 | Final query | 1 OR 2 | 29 hits | ||||
Search in Japanese database
Database: IPDL (Industrial property digital library), Japan
Date of search: 1900/01/01 to 2011/02/15
| S.No. | |
|
|
|
| |
|
|
|
|
Total patents: 1298 (Relevancy ~10%)
- F-Terms and theme used in search
| Japanese F-term search | Definition | ||
| Sr. No. | F- Term theme | 4H045 | Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof |
| 1 | F-term | DA15 | Peptide or protein characterised by function - Interferons |
| 2 | F-term | DA16 | Alpha-interferons |
| 3 | F-term | DA17 | Beta-interferons |
| 4 | F-term | DA18 | Gamma-interferons |
Scientific Literature Search
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
Sample patents
| |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Taxonomy
Sample Analysis
- Patents: The above sample patents were analysed according to the taxonomy.
Click here to download Sample analysis sheet on Interferon for treatment of Melanomas
- Scientific Literature:* Click here to download Melanoma Treatment Using IFN: sample articles
Patent Ranking
10 Sample Patents were ranked according to the patent focus.
- Patent Ranking Details
1 : Granted Patent & Focus in Independent Claim
2 : Granted Patent & Focus in Dependent Claim
3 : Published Patent & Focus in independent Claim
4 : Published Patent & Focus in Dependent claim
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
Clinical Trials
- Database: Clinical trials
- Searched on: Feb 25th, 2011
IP Activity Graphs Of Sample Patents
IP activity based on priority years
- Total of 10 Sample patents(basic patent number) were taken into consideration for the IP activity based on priority years.
IP activity based on publication years
- Total of 10 Sample patents(basic patent number) were taken into consideration for the IP activity based on publication years.
Geographical Distribution based on family members
- The geographical distribution is based on 10 sample patent numbers along with all their family members.
Market Report
Interferon types & Their Compositions
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
Interferon Types & Description Of Products
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
Global Revenue Data Of products
| S.No | Generic Name | Brand Name | Company | Global revenue ($ Million ) | ||
| 2008 | 2009 | 2010 | ||||
| 1 | Alpha IFN | Intron®,Roferon®-A | Schering Corporation | 38.4 | ||
| 2 | Beta IFN | Avonex | Biogen IDEC | 2518.4 | 2322.9 | 2202.6 |
| 3 | Gamma IFN | Actimmune® | Intermune | 29880 | 25428 | |
| 4 | Pegylated IFN | Peg Intron | Schering-Plough | 148.7 | ||
| 5 | Recombinant IFN | (Rebetron®, Rebetol®). | Schering Corporation | 36.1 | ||
Biosimilars Of Interferons
Biosimilar interferons are the major drug class in the recombinant non-glycosylated proteins market due to their extensive use in the treatment of various genetic and environmental disorders. The overall biosimilar market for interferons is categorized into the submarkets for alpha, beta, and gamma interferons, all of which are used extensively in the treatment of various conditions such as lymphoma, hairy cell leukemia, and multiple sclerosis.
While the interferon market growth may be inhibited by the side effects of each of its three drug categories, the market presents many opportunities to new entrants as only a few players currently operate in this relatively unfragmented market.
The global recombinant interferon market stood at $75.3 million in 2008 and is expected to reach $3.9 billion by 2014 at a CAGR of 82.9% from 2009 to 2014. The American biosimilar interferons market is expected to attain a market worth $1.5 billion by 2014 at a CAGR of 91.5%.Biosimilars ; Global Market
| |
|
|
| |
|
|
| |
|
|
| |
|
|